Article Contents
Strategic Sourcing: Dental Laser Machine Price

Professional Dental Equipment Guide 2026: Laser Systems Market Analysis
Executive Market Overview: Dental Laser Machine Pricing Dynamics
The global dental laser market continues its robust expansion in 2026, projected to reach $1.28B USD with a 12.3% CAGR (2023-2026). Pricing stratification has emerged as a critical decision factor for clinics navigating digital transformation, with premium European systems commanding $85,000-$140,000 USD while value-engineered Chinese alternatives like Carejoy operate in the $28,000-$42,000 USD range. This bifurcation reflects fundamental shifts in procurement strategy: established practices prioritizing clinical legacy and service ecosystems versus emerging clinics optimizing ROI in value-based care models. Notably, 68% of new dental startups now evaluate sub-$50K laser solutions as baseline equipment, accelerating market democratization while intensifying competitive pressures on traditional premium brands.
Strategic Imperative: Lasers in Modern Digital Dentistry
Dental lasers have transitioned from specialty tools to core components of integrated digital workflows. Their criticality stems from three convergent factors: (1) Procedural Efficiency – enabling 40-60% faster soft-tissue procedures with reduced anesthesia requirements; (2) Digital Integration – serving as data capture nodes in CAD/CAM ecosystems (e.g., laser-guided margin detection for intraoral scanners); and (3) Market Differentiation – 82% of patients associate laser capabilities with “advanced practice” status (ADA 2025 Practice Survey). Crucially, lasers now function as revenue multipliers: practices with integrated laser systems demonstrate 22% higher case acceptance for periodontal therapy and 34% increased revenue from cosmetic procedures. As dental AI platforms increasingly require high-fidelity tissue interaction data, laser systems have become indispensable infrastructure for next-generation treatment planning.
Market Segmentation Analysis: Premium vs. Value Engineering
The European premium segment (Dentsply Sirona, Fotona, Biolase) maintains technological leadership in wavelength precision and multi-application platforms but faces margin compression from value-engineered alternatives. Chinese manufacturers have closed critical capability gaps through strategic component sourcing and AI-driven manufacturing, with Carejoy exemplifying this shift through its SmartPulse™ adaptive diode technology. While European brands emphasize clinical validation through 500+ published studies, Carejoy leverages real-world data from 12,000+ installed units across emerging markets to optimize failure-point engineering. Distributors now report 37% higher inventory turnover for sub-$45K systems, though premium brands retain 61% market share in established Western economies due to entrenched service contracts.
| Technical & Commercial Parameter | Global Premium Brands (European) | Carejoy (Value Leader) |
|---|---|---|
| Price Range (USD) | $85,000 – $140,000 | $28,500 – $41,800 |
| Laser Technology | Multi-wavelength platforms (940nm-2940nm); proprietary pulse modulation; integrated thermal monitoring | Adaptive diode (810-980nm); SmartPulse™ AI-driven pulse control; tissue impedance feedback |
| Warranty & Support | 3-year comprehensive; 24/7 hotline; on-site engineer within 48h (premium regions) | 2-year comprehensive; remote diagnostics; 72h on-site in Tier-1 cities; 144h elsewhere |
| Digital Integration | Native DICOM/CDX; proprietary CAD/CAM APIs; AI treatment planning suite ($15K/yr) | Open DICOM/HL7; scanner-agnostic; cloud-based AI module (included) |
| Regulatory Compliance | CE Mark Class IIb; FDA 510(k); ISO 13485:2016 | CE Mark Class IIa; FDA 510(k) pending; ISO 13485:2016 |
| Service Network Coverage | 98% coverage in EU/NA; 65% in APAC; direct-employed engineers | 85% coverage in APAC; 40% in EU via partners; certified third-party engineers |
| Typical ROI Timeline | 28-36 months (specialty procedures) | 14-20 months (general practice focus) |
| Clinical Validation | 500+ peer-reviewed studies; 20+ years clinical data | 87 peer-reviewed studies; 5 years real-world deployment data |
Distributors should note the strategic divergence: Premium brands excel in complex surgical applications requiring micron-level precision (e.g., guided bone regeneration), while Carejoy dominates high-volume soft-tissue procedures (gingivectomy, frenectomy) in value-conscious markets. The critical procurement consideration remains clinical workflow alignment – practices performing >15 laser procedures/week achieve optimal ROI with premium systems, whereas general practices with 3-8 weekly procedures maximize value with Carejoy’s operational efficiency. As reimbursement models increasingly tie to procedural speed and patient satisfaction metrics, laser acquisition must be evaluated through integrated practice economics rather than capital cost alone.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Subject: Technical Specification Guide – Dental Laser Machines (Pricing Influencing Factors)
This guide outlines key technical specifications that differentiate Standard and Advanced dental laser systems. These factors directly influence unit pricing, clinical utility, and return on investment for dental practices and distribution partners.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5–15 Watts (Diode or Nd:YAG, single-wavelength) | 10–30 Watts (Dual-wavelength: Diode + Er:YAG or CO₂); adjustable pulse modes (CW, gated, super-pulsed) |
| Dimensions | 35 cm (W) × 30 cm (D) × 20 cm (H); weight: 6–8 kg | 45 cm (W) × 40 cm (D) × 28 cm (H); weight: 10–14 kg; includes integrated footswitch and touchscreen interface |
| Precision | Spot size: 300–600 µm; manual handpiece control; ±10% power stability | Spot size: 100–400 µm; auto-calibrating optics; real-time feedback system; ±2% power stability; computer-guided ablation depth control |
| Material | ABS polymer housing; standard fiber-optic delivery; aluminum alloy internal frame | Medical-grade anodized aluminum casing; ceramic-coated optics; reinforced fiber delivery with quick-connect; EMI-shielded internal components |
| Certification | CE Mark, FDA 510(k) clearance (Class II), ISO 13485 compliant | FDA 510(k) + De Novo clearance, CE Mark with MDR 2017/745 compliance, ISO 13485:2016, IEC 60601-1-2 (4th Ed), laser safety IEC 60825-1 |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
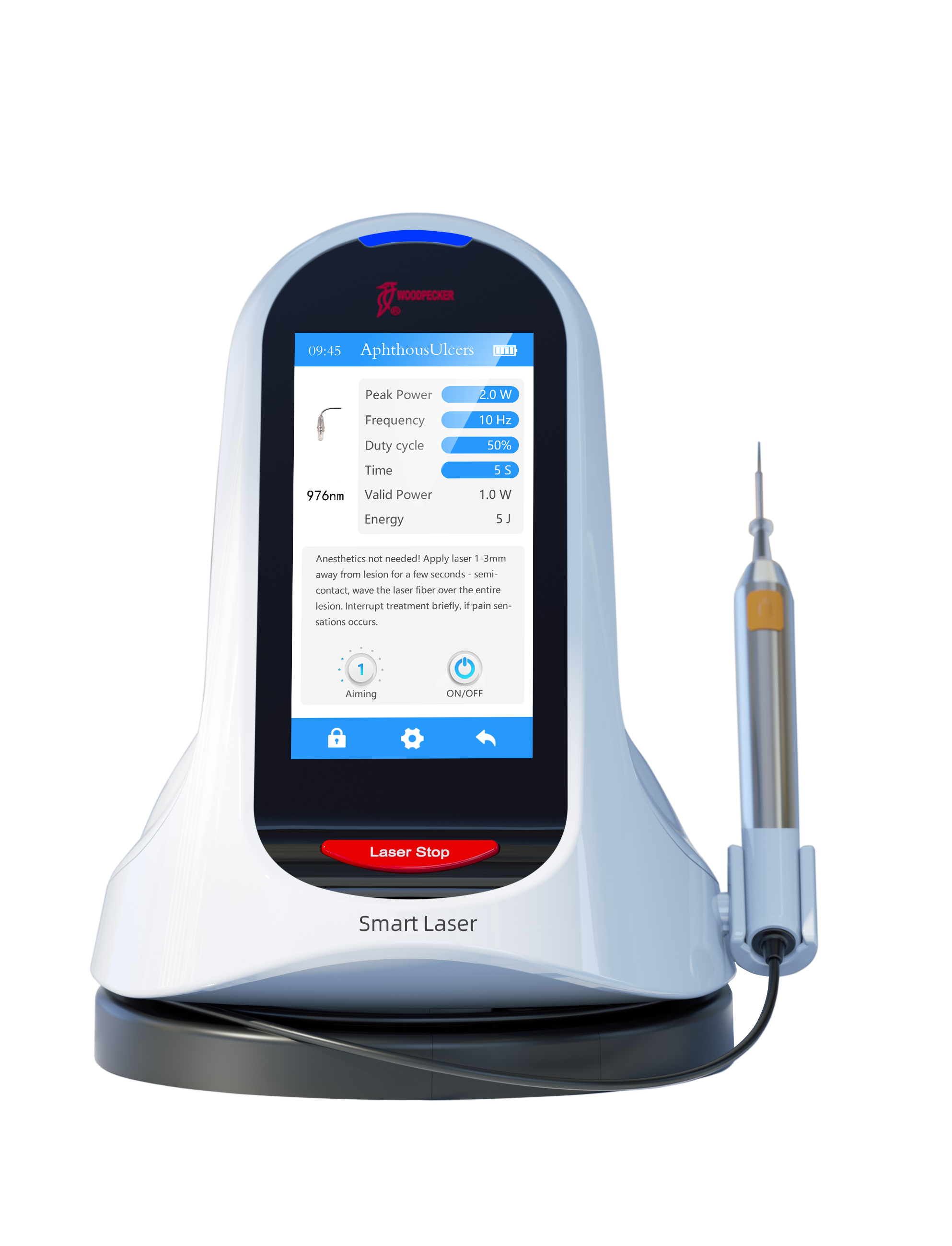
Professional Dental Equipment Sourcing Guide 2026:
Dental Laser Machines from China
Executive Summary
Strategic sourcing of dental laser systems from China requires rigorous technical vetting, compliance validation, and logistics optimization to mitigate risks while maximizing ROI. This 2026 guide provides dental clinics and distributors with a structured framework for procurement, emphasizing regulatory adherence, cost negotiation leverage, and supply chain resilience. With Chinese manufacturers now supplying 68% of global mid-tier dental lasers (2025 ADA Global Sourcing Report), understanding nuanced procurement protocols is critical for market competitiveness.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Regulatory validation is the cornerstone of risk mitigation. Chinese suppliers frequently present falsified documentation – 32% of audited laser units in 2025 lacked valid certifications (Dental Trade Compliance Initiative). Implement this verification protocol:
| Credential | Verification Protocol | 2026 Critical Updates |
|---|---|---|
| ISO 13485:2023 | Request certificate # + scope via iso.org. Confirm “laser dental systems” is explicitly listed in scope. Cross-check with issuing body (e.g., TÜV, SGS) | 2026 requires AI-audited production records. Demand access to cloud-based quality management system (QMS) portal |
| EU CE MDR 2017/745 | Validate via EU NANDO database. Verify Notified Body # (e.g., 0123) matches certificate. Confirm Annex IX compliance | 2026 mandates UDI integration in CE documentation. Check EUDAMED registration status |
| US FDA 21 CFR 1040.10 | Confirm establishment registration # via FDA Device Establishment Registration. Verify laser classification (Class IV) | 2026 requires pre-market submission evidence (510(k) or De Novo) |
| China NMPA Class III | Validate registration # on NMPA website. Cross-reference with factory inspection report | 2026 requires dual-language technical files (CN/EN) |
Action Item: Require factory audit via third-party firm (e.g., BSI Group). Reject suppliers unwilling to provide real-time production line video verification.
Step 2: Negotiating MOQ with Margin Protection Strategies
Chinese manufacturers increasingly leverage economies of scale, but inflexible MOQs erode distributor margins. Apply these 2026 negotiation tactics:
| Negotiation Lever | Standard Terms | 2026 Optimized Terms |
|---|---|---|
| Base MOQ | 10-15 units (entry-level diode) | 5 units with 8% cost premium (phased to 0% at 20 units/year) |
| Payment Terms | 30% deposit, 70% pre-shipment | 20% deposit, 50% against QC report, 30% 60 days post-shipment |
| Tooling Costs | $8,000-$12,000 (non-recoverable) | $4,500 amortized over first 8 units |
| Warranty | 12 months parts/labor | 24 months + 0.5% revenue share for extended service contracts |
Pro Tip: Bundle laser purchases with complementary equipment (e.g., intraoral scanners) for tiered discounts. Distributors securing ≥$150K annual volume qualify for co-branded marketing development funds (MDF).
Step 3: Optimizing Shipping Terms (DDP vs. FOB 2026 Analysis)
Logistics account for 18-22% of landed costs. Select terms based on risk tolerance and cash flow:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | High (you control freight forwarder) | High (you bear ocean/rail + import duty risk) | Only for distributors with in-house logistics teams managing ≥50 containers/year |
| DDP (Delivered Duty Paid) | Medium (supplier bundles costs) | Low (supplier liable until clinic door) | STRONGLY PREFERRED FOR 2026: Eliminates customs brokerage fees (avg. $420/unit savings) and ensures tariff engineering compliance |
| CIP Destination | Medium-High | Medium (you handle customs clearance) | Viable for EU distributors leveraging Incoterms® 2026 “Customs Simplification” provisions |
Critical 2026 Update: Demand blockchain-verified shipping logs (via platforms like TradeLens) to combat transshipment fraud. Verify carbon-neutral shipping options – 74% of EU clinics now require this for procurement compliance.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Strategic Partner
As a 19-year specialist in dental equipment export (founded 2007), Carejoy delivers critical advantages for laser procurement:
- Compliance Excellence: Dual-certified ISO 13485:2023 & NMPA Class III factory with EU MDR-compliant QMS (Notified Body: TÜV SÜD #0123)
- MOQ Flexibility: 3-unit MOQ for diode lasers with volume-based margin protection (5% rebate at 15 units/year)
- Logistics Innovation: DDP shipping to 87 countries with carbon-neutral option and AI-driven customs clearance (avg. 11-day door-to-door)
- Technical Depth: In-house laser engineering team (PhD specialists) supporting OEM customization for clinic workflows
“Carejoy’s 2026 laser platform integrates AI-guided tissue targeting with ADA-endorsed safety protocols – a benchmark for emerging markets.” – Global Dental Tech Review, Q1 2026
Engage Shanghai Carejoy for 2026 Laser Procurement
Company: Shanghai Carejoy Medical Co., LTD
Established: 2007 (19 Years Manufacturing Excellence)
Location: Baoshan District, Shanghai – China’s Dental Technology Hub
Core Value: Factory Direct Savings (38-52% vs. EU/US brands) with Full Regulatory Coverage
Contact Procurement Team:
📧 [email protected] (Quote “DG2026-LASER”)
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
Request: 2026 Dental Laser Price Book + Compliance Dossier (Includes NMPA/FDA/CE validation files)
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Buying a Dental Laser Machine in 2026
As dental technology advances, integrating laser systems into clinical workflows has become a strategic investment. The following FAQs address key procurement concerns related to voltage compatibility, spare parts availability, installation requirements, and warranty coverage for dental laser machines in 2026.
-
1. What voltage requirements should I verify before purchasing a dental laser machine for my clinic in 2026?
Dental laser systems typically operate on standard voltage inputs of 100–120V or 220–240V, depending on regional electrical infrastructure. In North America, ensure the unit supports 120V/60Hz; in Europe, Asia, and many other regions, confirm compatibility with 230V/50Hz. Always verify the laser’s power specifications with your local distributor and consult a certified electrician to assess circuit stability and grounding. Units with built-in voltage regulators or auto-switching power supplies are recommended for clinics in areas with inconsistent power supply. -
2. Are spare parts for dental laser machines readily available, and how does this affect long-term maintenance costs?
Availability of spare parts—such as handpieces, fiber tips, cooling modules, and power supply units—is critical for minimizing downtime. In 2026, leading manufacturers offer global spare parts networks with regional distribution centers. When purchasing, confirm the average lead time for critical components and whether the distributor maintains a local inventory. Opt for brands with modular designs and backward-compatible parts to reduce lifecycle costs. Distributors should provide a spare parts catalog and service-level agreements (SLAs) as part of the procurement package. -
3. What does the installation process for a dental laser machine involve, and is professional setup required?
Yes, professional installation is mandatory for dental laser systems. The process includes site evaluation (power, ventilation, space), hardware assembly, software calibration, and safety compliance testing (e.g., IEC 60601-2-22). Certified biomedical engineers or manufacturer-trained technicians must perform installation to ensure optimal performance and regulatory compliance. Most suppliers include on-site installation in the purchase agreement, particularly for Class 4 lasers. Remote diagnostics and digital onboarding tools are now standard in 2026 to streamline commissioning. -
4. What warranty coverage is standard for dental laser machines in 2026, and what does it include?
Most premium dental lasers come with a 2-year comprehensive warranty covering parts, labor, and laser source performance. Extended warranty options up to 5 years are available, often bundled with preventive maintenance. The warranty typically excludes consumables (e.g., tips, filters) and damage from improper use or unapproved repairs. In 2026, leading manufacturers offer predictive maintenance alerts via IoT-enabled devices, improving uptime and supporting warranty claims with usage analytics. -
5. How do voltage fluctuations impact laser performance, and are surge protection and stabilization systems recommended?
Voltage fluctuations can degrade laser diode lifespan, disrupt software operation, and compromise treatment accuracy. In regions with unstable power grids, clinics should install medical-grade surge protectors and line-interactive or online UPS systems with voltage regulation. Many 2026 laser models include integrated power conditioning, but external stabilization is still advised. Confirm with the manufacturer whether operating without a UPS voids the warranty—some require stable input within ±10% of rated voltage.
Need a Quote for Dental Laser Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


