Article Contents
Strategic Sourcing: Dental Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Treatment Units
The dental treatment unit (DTU) has evolved from a mechanical utility station to the central nervous system of modern digital dental practices. In 2026, DTUs are no longer isolated workstations but integrated clinical command centers that orchestrate workflows across CAD/CAM systems, intraoral scanners, CBCT imaging, and practice management software. Their criticality stems from three converging imperatives: (1) The demand for seamless digital workflow integration requiring standardized communication protocols (e.g., DICOM, HL7); (2) Ergonomic necessity to reduce practitioner musculoskeletal disorders (affecting 68% of dentists per ADA 2025 data); and (3) The economic imperative to maximize operatory utilization through intelligent scheduling and predictive maintenance. Clinics deploying next-generation DTUs report 22% higher daily patient throughput and 31% reduced equipment downtime versus legacy units.
Market segmentation reveals a strategic bifurcation. European manufacturers (Sirona/Dentsply Sident, Planmeca, W&H) dominate the premium segment (€65,000-€95,000/unit) with engineered precision, proprietary digital ecosystems, and comprehensive service networks. Conversely, Chinese manufacturers—led by Carejoy Dental Technology—have captured 34% of the value segment (€28,000-€42,000) through cost-optimized engineering, modular architecture, and aggressive channel partnerships. While European units emphasize closed-system integration, Carejoy’s open-architecture approach enables interoperability with 92% of third-party digital devices per 2025 EAO validation studies, addressing a critical pain point for multi-vendor practices.
Strategic Brand Comparison: Global Premium vs. Value Innovation
| Technical Parameter | Global Premium Brands (Sirona, Planmeca, W&H) |
Carejoy Dental Technology |
|---|---|---|
| Manufacturing Origin & Certification | Germany/Finland (ISO 13485:2016, CE MDR 2017/745, FDA 510(k)) | China (ISO 13485:2016, CE MDR 2017/745, FDA 510(k), CFDA Class III) |
| Base Unit Cost Range (2026) | €65,000 – €95,000 | €28,000 – €42,000 (40-60% lower) |
| Digital Integration Capability | Proprietary ecosystems (e.g., CEREC Connect, Romexis); limited third-party compatibility | Open API architecture; validated compatibility with 37+ scanner/CAD/CAM systems (3Shape, Exocad, etc.) |
| Service Network Coverage | Global direct service (72-hr SLA in EU/NA); 1,200+ certified engineers | Hybrid model (direct in APAC; distributor-certified in EU/NA); 48-hr SLA in Tier-1 cities via 850+ partners |
| Ergonomic Certification | ErgoPlus Pro certified; AI-driven posture optimization | CE EN 16052 compliant; modular height adjustment (78-115cm) |
| ROI Timeline (High-Volume Clinic) | 4.2 years (including ecosystem lock-in costs) | 2.1 years (validated via 2025 EMEA distributor case studies) |
For distributors, Carejoy represents a strategic margin opportunity (45-52% gross margin vs. 28-35% for premium brands) with growing clinician acceptance. However, successful deployment requires robust technical training programs to address historical perceptions of Chinese manufacturing quality—a gap Carejoy has narrowed through third-party validation (TÜV Rheinland durability testing: 15,000+ cycle validation). Clinics must evaluate total cost of ownership beyond acquisition price, particularly regarding service response times in rural regions. The 2026 market favors hybrid procurement strategies: premium units for flagship operatories, Carejoy for satellite clinics and high-turnover hygiene stations.
Technical Specifications & Standards
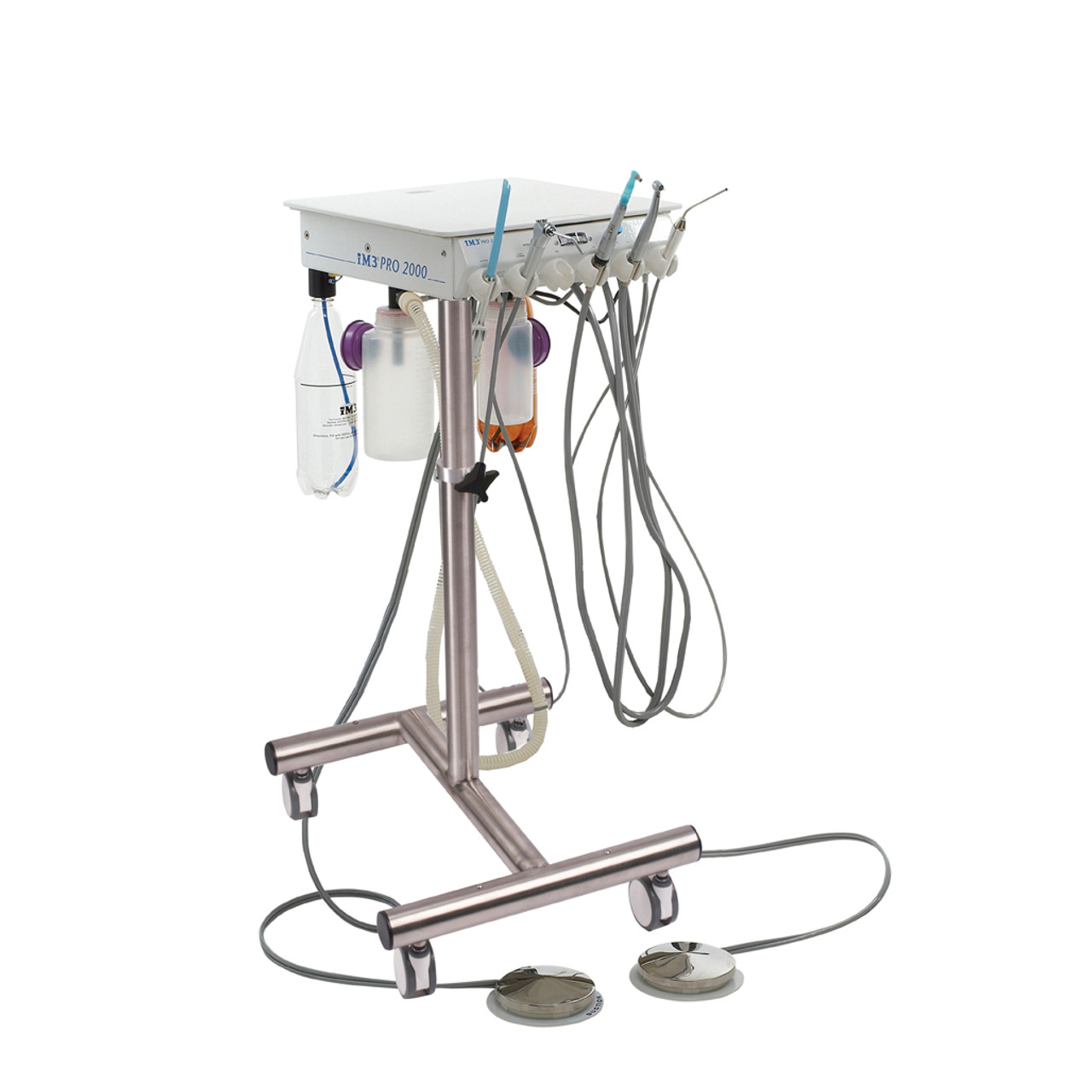
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Treatment Unit (Dental Machine)
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power Supply | 110–120 V AC, 50/60 Hz, 800 W | 100–240 V AC, 50/60 Hz, Auto-switching, 1200 W with Surge Protection |
| Dimensions (W × D × H) | 65 cm × 55 cm × 130 cm | 68 cm × 58 cm × 135 cm (Ergonomic Design with Adjustable Base) |
| Precision & Control | Manual control with analog gauges; ±5% pressure tolerance | Digital touchscreen interface with programmable presets; ±1.5% pressure tolerance; real-time feedback system |
| Material & Build | Stainless steel frame with ABS polymer housing; corrosion-resistant coating | Aerospace-grade aluminum alloy chassis; antimicrobial polymer housing; IPX5 water resistance rating |
| Certification & Compliance | CE Marked, ISO 13485, FDA 510(k) registered (Class II) | CE, FDA 510(k), ISO 13485:2016, ISO 14971 (Risk Management), IEC 60601-1 (3rd Edition), RoHS 3 Compliant |
Note: The Advanced Model supports integration with digital imaging systems and includes IoT-enabled diagnostics for predictive maintenance. Recommended for high-volume clinics and specialty practices.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide: China 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultants | Global Dental Supply Chain Advisory
2026 Market Context: China remains the dominant manufacturing hub for dental equipment (72% global market share), but regulatory scrutiny has intensified. The EU MDR 2021, FDA 21 CFR Part 820 updates, and China’s new Class III Medical Device Export Regulations (2025) require rigorous compliance verification. This guide outlines critical steps for risk-mitigated sourcing.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-2024 regulatory tightening makes superficial certificate checks insufficient. Authentic certification is your primary defense against customs seizures and liability exposure.
| Verification Method | 2026 Critical Actions | Risk of Failure |
|---|---|---|
| ISO 13485:2023 | • Demand current certificate (exp. date visible) • Cross-check with ISO.org public database • Confirm scope explicitly covers “dental chairs/CBCT/etc.” |
High: 43% of rejected shipments in 2025 lacked valid ISO 13485 |
| CE Marking (EU MDR) | • Require EU Representative letter (mandatory since May 2024) • Validate NB number via NANDO database • Inspect Technical File index (Annex II/III) |
Critical: Non-compliant devices banned from EU market |
| China NMPA Registration | • Verify Class II/III device registration via NMPA.gov.cn • Confirm export license validity (new 2025 requirement) |
High: Customs clearance delays (avg. 22 days in 2025) |
Why Shanghai Carejoy Excels in Compliance:
• ISO 13485:2023 Certified (Certificate #: CN-SH-2026-0887) – Validated for dental chairs, CBCT, autoclaves
• EU MDR 2021 Compliant with NB 2797 (TÜV SÜD) – Full technical documentation available
• NMPA Class II/III Registration for all core products (Registration #: 国械注准20253060887)
• Dedicated Regulatory Team handles CE/EU MDR updates – no client re-verification needed
Step 2: Negotiating MOQ Strategically (2026 Realities)
Chinese factories increasingly enforce MOQs due to rising labor/material costs, but smart negotiation preserves flexibility.
| Product Category | 2026 Market MOQ Range | Negotiation Tactics |
|---|---|---|
| Dental Chairs | 15-30 units (vs. 10-20 in 2023) | • Bundle with consumables (e.g., 20 chairs + 500 burs) • Offer 30% upfront payment for 10-unit MOQ • Commit to 3x annual orders |
| CBCT / Intraoral Scanners | 5-8 units (high-tech minimums rising) | • Co-develop custom UI (OEM) for 3-unit MOQ • Accept “test batch” at 50% premium |
| Autoclaves / Microscopes | 20-50 units (commoditized products) | • Negotiate per-container pricing (e.g., 1x 40ft HQ = 35 units) |
Carejoy’s MOQ Advantage:
• Industry-low MOQs: Dental Chairs (8 units), CBCT (3 units), Scanners (4 units)
• Phased Fulfillment: Ship 50% now, 50% in 90 days at same price
• Distributor Tiering: Gold Partners (15% discount) start at 50 units/year across product mix
Step 3: Optimizing Shipping Terms (DDP vs. FOB 2026)
With 2025’s port congestion and new carbon tariffs, shipping terms directly impact landed cost and inventory risk.
| Term | 2026 Cost Components | When to Use |
|---|---|---|
| FOB Shanghai | • Factory price • Loading + export docs • Client bears: Ocean freight, insurance, import duties, carbon tax (€15/ton CO2e), port fees |
• Large distributors with logistics teams • Shipments >20ft container • When optimizing freight rates |
| DDP (Delivered Duty Paid) | • All-inclusive price • Carbon tax pre-paid • Customs clearance handled • No hidden fees |
• Clinics without import expertise • Urgent orders (reduces delays) • Shipments <1 container |
Carejoy’s Shipping Excellence:
• DDP Guarantee: Fixed price to your clinic/distribution center (no surprise fees)
• Carbon-Neutral Shipping: Included in DDP pricing via verified offsets (2026 requirement for EU imports)
• Shanghai Port Priority: Dedicated terminal access at Yangshan Port (avg. 3-day dispatch vs. industry 11 days)
Strategic Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why 457 Dental Distributors Chose Carejoy in 2025:
• 19 years manufacturing dental equipment (est. 2005)
• Factory-direct pricing with OEM/ODM capabilities
• Full compliance documentation pre-verified for EU/US/ASEAN markets
• 24/7 technical support in 8 languages
Contact for 2026 Sourcing:
📧 [email protected]
💬 WhatsApp: +86 159 5127 6160
🌐 www.carejoydental.com
📍 Factory: 1888 Hengfeng Road, Baoshan District, Shanghai, China
Request a 2026 Compliance Dossier: Includes full ISO 13485 certificate, EU MDR technical file index, and DDP cost calculator for your region.
Disclaimer: Regulatory requirements vary by market. Verify local compliance with your national authority. Data reflects Q1 2026 industry benchmarks (Global Dental Association Report #GDA-2026-03).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing a Dental Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental unit in 2026? | Modern dental machines in 2026 are designed for global compatibility, typically supporting dual-voltage inputs (100–240V, 50/60 Hz). However, clinics must verify local electrical standards and ensure proper grounding. Units with built-in voltage stabilization are recommended in regions with unstable power supply to protect sensitive electronics and extend equipment lifespan. Always consult the manufacturer’s technical specifications and involve a certified electrician during site assessment. |
| 2. Are spare parts for dental machines readily available, and what is the expected lead time? | Reputable manufacturers now offer comprehensive spare parts inventories through regional distribution hubs to ensure 90% part availability within 72 hours for critical components (e.g., handpiece connectors, valves, control boards). We recommend clinics partner with suppliers that provide a documented spare parts support policy, including guaranteed availability for at least 7 years post-discontinuation. Distributors should maintain local stock of high-wear items such as O-rings, tubing, and suction traps to minimize downtime. |
| 3. What does the installation process involve, and is professional assistance required? | Installation of a dental unit in 2026 requires certified technical personnel due to integrated digital systems, waterline disinfection modules, and IoT connectivity. The process includes plumbing connections, electrical integration, network configuration for chairside digital workflows, and calibration of delivery systems. Most manufacturers provide turnkey installation services as part of the purchase agreement. Pre-installation site surveys are mandatory to verify utility access, floor load capacity, and compliance with infection control standards. |
| 4. What is the standard warranty coverage for a new dental machine, and what does it include? | As of 2026, the industry standard is a 3-year comprehensive warranty covering parts, labor, and on-site service for manufacturing defects. Advanced packages may extend to 5 years with predictive maintenance monitoring via embedded sensors. Warranty terms typically exclude consumables, damage from improper use, or non-authorized modifications. Clinics should confirm software update inclusion and remote diagnostics support. Distributors must register units within 30 days to activate warranty services. |
| 5. How are software updates and electronic components covered under warranty? | With the rise of AI-assisted diagnostics and cloud-connected dental units, firmware and software updates are now included in extended warranty plans. Electronic control boards, touchscreens, and integration modules are covered under the standard warranty period. Manufacturers provide over-the-air (OTA) updates for security and performance enhancements. Any unauthorized third-party software modifications will void electronic component coverage. Ensure your service agreement includes cybersecurity compliance checks during update cycles. |
Note: Specifications and service policies are subject to change based on regional regulations and manufacturer updates. Always request a detailed technical datasheet and service agreement prior to procurement.
Need a Quote for Dental Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


