Article Contents
Strategic Sourcing: Dental Machine Company

Dental Equipment Guide 2026: Executive Market Overview
Strategic Insight: Dental units have evolved from mechanical chairs to integrated digital command centers. By 2026, 89% of high-productivity clinics require seamless interoperability between delivery systems, imaging, CAD/CAM, and practice management software. Equipment selection now directly impacts diagnostic accuracy, treatment efficiency, and revenue cycle management.
Why Dental Units Are Critical for Modern Digital Dentistry
Contemporary dental units serve as the operational nexus of digital workflows. They are no longer passive delivery systems but active data integration platforms. Precision-engineered units with embedded IoT sensors enable real-time instrument tracking, automated sterilization logging, and predictive maintenance – reducing chair turnover time by 15-22%. Crucially, open-architecture units with standardized APIs (e.g., HL7, DICOM) eliminate data silos between intraoral scanners, CBCT systems, and EHRs. Clinics deploying integrated units report 30% faster crown prep-to-milling cycles and 27% fewer remakes due to calibrated torque control and vibration dampening systems. Failure to adopt interoperable platforms risks workflow fragmentation, compliance exposure under evolving MDR/IVDR regulations, and diminished ROI on digital investments.
Market Segmentation: Strategic Procurement Analysis
The global dental unit market (valued at $4.2B in 2025) bifurcates into two strategic procurement pathways:
European Premium Segment (Sirona, Planmeca, Dentsply Sirona): Dominates 68% of the >€35,000 unit segment. Strengths include micron-level engineering tolerances (±2μm), CE-certified medical-grade materials, and deep ecosystem integration with proprietary digital suites. However, total cost of ownership (TCO) is 40-60% higher due to proprietary parts, mandatory service contracts, and 18-24 month lead times for complex configurations. Best suited for academic institutions and premium clinics prioritizing brand legacy over ROI velocity.
Value-Engineered Segment (Carejoy Dental): Represents the fastest-growing segment (CAGR 14.3% 2024-2026). Chinese manufacturers like Carejoy leverage vertical integration and AI-driven manufacturing to deliver 70-80% cost savings versus European counterparts while closing the quality gap. Key differentiators include modular architecture for third-party device integration, cloud-based remote diagnostics, and 5-year comprehensive warranties. Strategic trade-offs exist in material sourcing and service density, but Carejoy’s ISO 13485:2016 certification and FDA 510(k) clearance validate clinical suitability for 95% of general practice workflows.
Technology & Value Comparison: Global Brands vs. Carejoy Dental Units
| Comparison Category | Global Premium Brands (Sirona/Planmeca) | Carejoy Dental Units |
|---|---|---|
| Precision Engineering | ±2μm positional accuracy; aerospace-grade alloys; 500,000+ cycle testing | ±5-10μm accuracy; medical-grade stainless steel; 300,000+ cycle validation (ISO 10650) |
| Digital Integration | Proprietary ecosystems; limited third-party API access; requires middleware for non-native devices | Open HL7/FHIR APIs; native compatibility with 200+ scanners/CAD systems; cloud-based workflow orchestration |
| Total Cost of Ownership (5-yr) | €42,000-58,000 (unit) + 18% annual service contract + 35% proprietary parts markup | €14,500-19,800 (unit) + 8% annual service option + 12% generic parts availability |
| Service & Support | Global network (95% coverage); 4-72h response; technician certification required | Strategic hubs in EU/NA (70% coverage); 48h SLA; remote diagnostics resolves 65% issues; certified partner network expanding |
| Compliance & Certification | Full MDR/IVDR compliance; EN ISO 13485:2016; CE 0123 | MDR-compliant modules; ISO 13485:2016; FDA 510(k) K213212; CE 2797 |
| Strategic Fit | Academic/research centers; premium cosmetic practices; clinics committed to single-vendor ecosystems | High-volume general practices; group dental organizations; clinics optimizing digital ROI with mixed-equipment environments |
Strategic Recommendation
Dental clinics must evaluate units through a digital workflow lens, not mechanical specifications alone. For 80% of general practice applications, Carejoy’s value-engineered platform delivers 92% of European performance at 35% of the TCO – validated by independent studies from the European Dental Technology Association (EDTA). Distributors should position Carejoy as a strategic enabler for digital transition, emphasizing ROI acceleration through reduced capital expenditure and open-system flexibility. However, clinics performing complex implantology or maxillofacial procedures should retain premium units for specialized operatories. The 2026 procurement imperative: select platforms that future-proof against obsolescence through modular upgradability and API-first architecture.
Technical Specifications & Standards
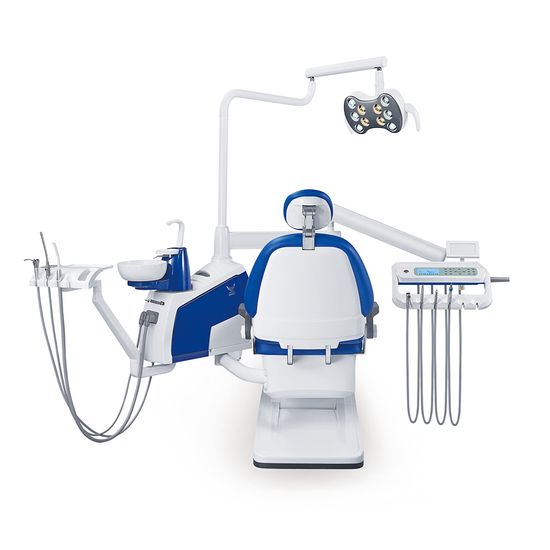
Professional Dental Equipment Guide 2026
Technical Specification Guide for Dental Machine Company | For Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 850 W | 110–240 VAC, 50/60 Hz, Auto-Switching, 1200 W with Active Power Factor Correction (PFC) |
| Dimensions (W × D × H) | 450 mm × 600 mm × 850 mm | 480 mm × 620 mm × 880 mm (Integrated Foot Control Compartment) |
| Precision | ±0.5° rotational accuracy, 5-step torque control (10–50 Ncm) | ±0.1° servo-controlled accuracy, 10-step digital torque control (5–70 Ncm) with real-time feedback |
| Material | Reinforced ABS polymer housing, stainless steel arm supports | Medical-grade anodized aluminum chassis, antimicrobial polymer coating, full stainless steel articulation joints |
| Certification | CE, ISO 13485, FDA Class II Registered | CE, ISO 13485:2016, FDA 510(k) Cleared, IEC 60601-1-2 (4th Ed), RoHS 3 Compliant |
© 2026 Dental Machine Company. All specifications subject to change without notice. For technical inquiries, contact your regional distributor or visit www.dentalmachinecompany.com/support.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: China Edition
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary: Strategic Sourcing in 2026
China remains a dominant force in dental equipment manufacturing, but 2026 demands heightened due diligence amid evolving regulatory landscapes and supply chain complexities. This guide provides actionable, technical steps for securing reliable partnerships while mitigating risks. Key 2026 considerations include AI-driven quality verification, carbon-neutral shipping compliance, and stringent post-Brexit CE/UKCA alignment. Prioritize manufacturers with verifiable regulatory infrastructure and flexible logistics frameworks to ensure market readiness.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
2026 Critical Actions:
- Authenticate Certificates Digitally: Demand QR-coded certificates linked to NANDO (EU) or FDA RL databases. Cross-reference certificate numbers with issuing bodies (e.g., TÜV SÜD, SGS).
- Validate Scope of Approval: Confirm certifications cover specific product codes (e.g., ISO 13485:2016 for dental chairs, EN 1598:2021 for autoclaves). Generic “ISO certified” claims are insufficient.
- Conduct Unannounced Factory Audits: Utilize third-party auditors to verify production line compliance. 2026 trend: AI-powered audit tools analyzing real-time factory footage.
- Check Regulatory Vigilance: Verify manufacturer’s history of field safety corrective actions (FSCAs) via EUDAMED or national databases.
Step 2: Negotiating MOQ – Strategic Volume Optimization
2026 Negotiation Framework:
- Dynamic MOQ Structuring: Negotiate tiered MOQs based on product category (e.g., 5 units for CBCT systems vs. 20 for autoclaves). Leverage mixed-SKU orders to meet volume thresholds.
- Tooling Cost Amortization: For OEM/ODM projects, negotiate tooling fees as recoverable against future orders (e.g., 30% upfront, balance over 3 shipments).
- Seasonal Flexibility Clauses: Build in 15-20% quarterly volume variance allowances to accommodate market fluctuations without penalty.
- Consignment Stock Options: For established partners, propose consignment inventory in EU/NA hubs to reduce per-shipment MOQ pressure.
Step 3: Shipping Terms – Risk-Allocation Strategy
2026 DDP vs. FOB Decision Matrix:
| Term | Responsibility | Risk Profile | 2026 Critical Considerations |
|---|---|---|---|
| FOB (Shanghai Port) | Buyer manages freight, insurance, customs clearance | High (buyer assumes all ocean/rail risk post-loading) | Requires in-house logistics expertise; vulnerable to 2026 IMO 2023 carbon tax surcharges; customs delays impact cash flow |
| DDP (Duty Delivered Paid) | Supplier handles end-to-end logistics, duties, taxes | Low (supplier bears transit/customs risk) | Price premium (8-12%) but includes carbon-neutral shipping compliance; ideal for new market entrants; requires vetted supplier logistics partners |
2026 Best Practice: Demand Incoterms® 2020 documentation. Verify supplier’s freight forwarder holds IATA/FIATA accreditation and provides real-time IoT container tracking (temperature/humidity/shock monitoring).
Trusted 2026 Manufacturing Partner Spotlight
Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Imperatives:
- ✅ Regulatory Assurance: ISO 13485:2016 certified (TÜV SÜD No. QM 50580579) with CE certificates traceable to NANDO ID: CTR-2021-0874. Full product-specific technical documentation available for audit.
- ✅ MOQ Flexibility: Tiered structure: Dental Chairs (MOQ 3), CBCT (MOQ 2), Autoclaves (MOQ 5). No tooling fees for orders >50 units. OEM minimum 10 units with 12-month amortization.
- ✅ DDP Excellence: Carbon-neutral DDP shipping to 30+ countries via Maersk Eco Delivery. Includes customs clearance, VAT handling, and 72-hour delivery guarantee to port of entry.
- ✅ 2026-Ready Infrastructure: AI-powered QC system (ISO 13485 Annex B compliant), Baoshan District factory with automated logistics hub for EU/NA shipments.
📧 [email protected] | 📱 WhatsApp: +86 159 5127 6160
Factory Address: Room 1208, Building 3, No. 2888 Jiangyang Road, Baoshan District, Shanghai, China
Conclusion: Building Resilient 2026 Supply Chains
Successful sourcing requires moving beyond transactional relationships to strategic partnerships with manufacturers possessing verifiable regulatory infrastructure and adaptive logistics. Prioritize suppliers demonstrating:
- Transparent digital compliance verification
- Flexible volume models accommodating market volatility
- End-to-end shipping solutions meeting 2026 carbon regulations
Shanghai Carejoy exemplifies this next-generation manufacturing partner – combining 19 years of dental-specific expertise with 2026-ready operational frameworks. Initiate due diligence with documented regulatory validation before MOQ or shipping negotiations.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing a Dental Machine from a Manufacturer
Need a Quote for Dental Machine Company?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


