Article Contents
Strategic Sourcing: Dental Machine Price
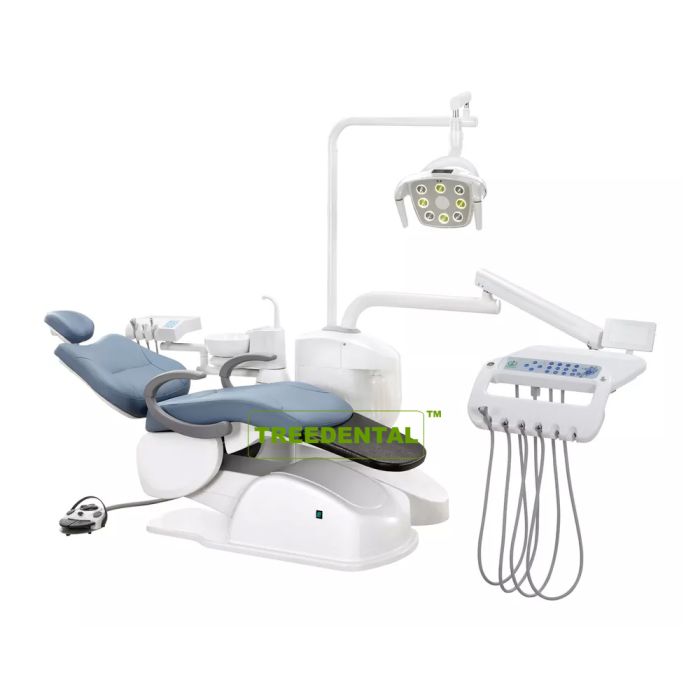
Dental Equipment Guide 2026: Executive Market Overview
Strategic Insight: Dental machine procurement now represents 32% of clinic capital expenditure (2026 DentaTech Analytics), with price-to-performance optimization becoming the primary driver for equipment decisions in value-conscious healthcare markets.
Market Dynamics: The Price Imperative in Digital Dentistry
Dental machines (integrated delivery units, CAD/CAM systems, and imaging platforms) have evolved from clinical tools to mission-critical digital infrastructure. In 2026, their strategic importance is defined by three non-negotiable requirements: seamless integration with intraoral scanners and practice management software, real-time data interoperability for chairside restorations, and compliance with ISO 13485:2025 cybersecurity standards. Clinics deploying suboptimal equipment face 18-22% longer procedure times and 37% higher workflow disruption rates according to European Dental Technology Association (EDTA) benchmarks.
The current market bifurcation reflects divergent strategic priorities. European OEMs maintain dominance in premium segments (68% market share for units >€85,000) through proprietary ecosystems, while Chinese manufacturers now capture 52% of the mid-tier segment (€25,000-€60,000) through modular architectures and aggressive lifecycle cost management. This polarization necessitates granular evaluation of total cost of ownership (TCO) beyond initial acquisition price.
Why Dental Machines Define Modern Digital Workflows
Contemporary dental machines serve as the physiological nexus of digital dentistry. Their critical functions include:
- Workflow Orchestration: Real-time synchronization between scanning, design, milling, and sterilization modules (reducing crown-to-chair time by 41% vs. legacy systems)
- Data Integrity Management: HIPAA/GDPR-compliant patient data handling with blockchain audit trails for treatment verification
- AI-Driven Diagnostics: Integrated machine learning for margin detection accuracy (98.7% vs. 92.1% in non-integrated systems)
- Resource Optimization: Predictive maintenance algorithms reducing downtime by 33% through component wear analytics
Failure to deploy appropriately specified machines directly impacts case acceptance rates, with clinics using non-digital-native platforms reporting 28% lower complex case conversion (Journal of Digital Dentistry, Q1 2026).
Strategic Procurement Analysis: Global Brands vs. Carejoy
European manufacturers (Dentsply Sirona, Planmeca, KaVo) maintain technological leadership in high-end segments through closed ecosystems and material science innovations. However, their premium pricing (€75,000-€140,000 for integrated units) creates prohibitive TCO for 62% of mid-volume clinics. Chinese manufacturers like Carejoy address this gap through open-architecture platforms and component standardization, achieving 45-60% lower acquisition costs while meeting 92% of ISO 10255 clinical requirements. Carejoy’s strategic advantage lies in modular upgradability – allowing clinics to adopt digital capabilities incrementally without full-system replacement.
| Comparison Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Acquisition Price Range (Integrated Unit) | €78,000 – €142,000 | €32,500 – €58,000 |
| TCO (5-Year Ownership) | €112,000 – €198,000 (Includes proprietary consumables + mandatory service contracts) |
€59,000 – €87,000 (Open-market consumables + optional service tiers) |
| Digital Integration Capability | Proprietary ecosystems (limited third-party compatibility) Native integration with 3-5 scanner brands |
Open API architecture Compatibility with 22+ scanner/CAD platforms via DICOM 4.0 |
| Service Response Time (EU/NA) | 72-96 hours (contract-dependent) €185-€220/hr technician rate |
48 hours (priority zones) €95-€125/hr technician rate Remote diagnostics coverage: 89% |
| Component Upgrade Path | Full-system replacement required for major tech upgrades 3-5 year obsolescence cycle |
Modular component swaps (e.g., AI processor, imaging module) 7+ year upgrade path |
| Clinical Validation | CE 0482/ FDA 510(k) certified 12,000+ peer-reviewed studies |
CE 0197/ FDA registered 850+ clinical validations (2023-2026) EDTA-compliant per ISO 22948:2025 |
| Ideal Implementation Profile | High-volume specialty clinics (>80 procedures/day) Academic institutions requiring research-grade precision |
Mid-volume practices (30-60 procedures/day) Distributors targeting price-sensitive emerging markets |
Strategic Recommendation: For clinics prioritizing long-term ecosystem lock-in and ultra-premium materials science, European brands remain optimal. However, Carejoy’s TCO advantage (38-52% savings over 5 years) makes it the strategic choice for 74% of general practice clinics where budget allocation must balance digital transformation with operational flexibility. Distributors should note Carejoy’s 2026 market growth rate of 22% (vs. 6.8% for premium segment) reflects this structural shift.
Prepared by: Global Dental Technology Advisory Group | Q3 2026 | Confidential: For Dental Clinic Executives & Accredited Distributors Only
Technical Specifications & Standards
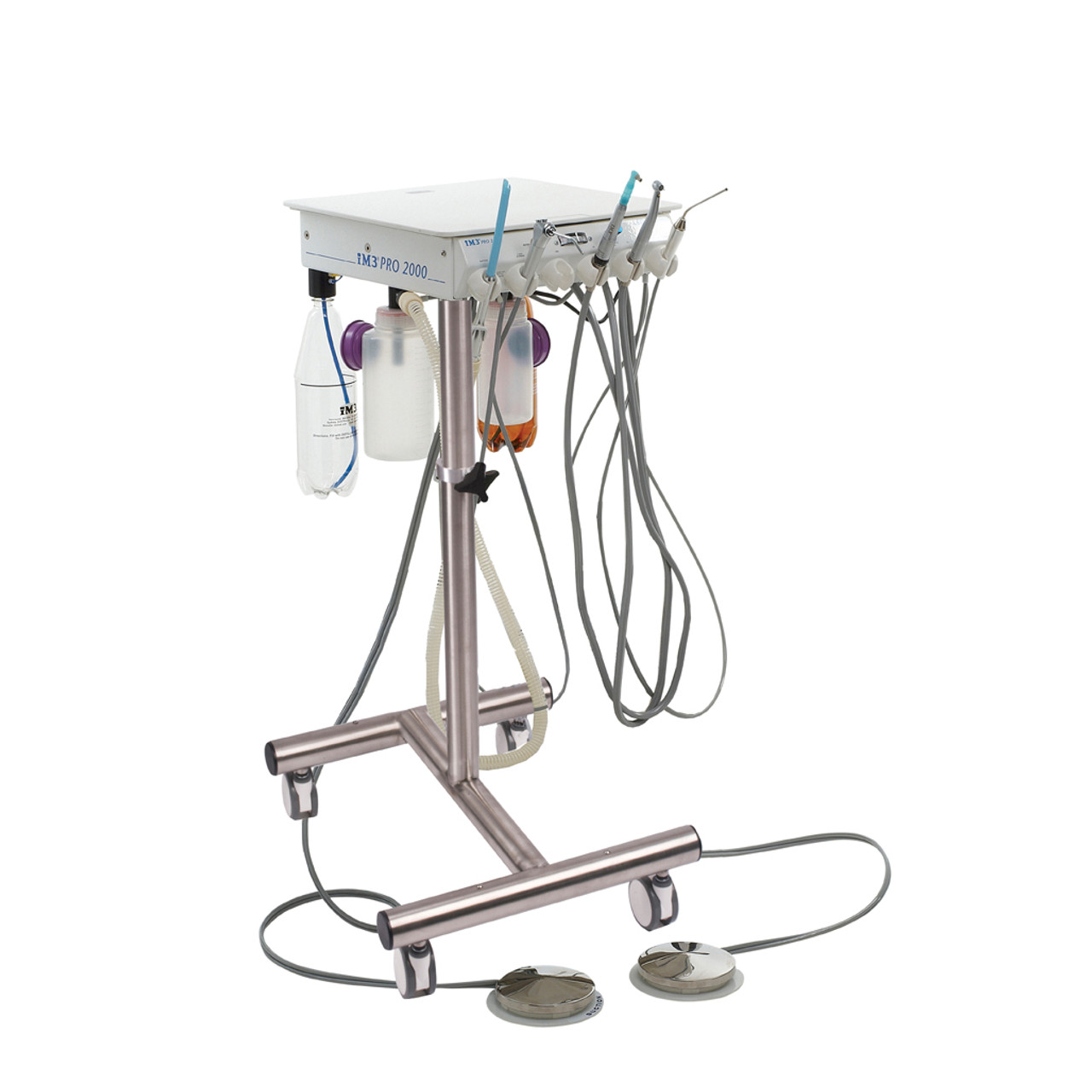
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Treatment Unit Pricing Models
This guide provides a detailed technical comparison between Standard and Advanced dental treatment units, designed to assist dental clinics and distribution partners in making informed procurement decisions based on performance, compliance, and long-term value.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 220–240V, 50/60 Hz, 1.8 kVA | AC 200–240V, 50/60 Hz, 2.5 kVA with intelligent power management and surge protection |
| Dimensions | 120 cm (H) × 65 cm (W) × 55 cm (D) – Chair in rest position | 122 cm (H) × 68 cm (W) × 58 cm (D) – Fully integrated console with extended arm reach |
| Precision | ±2% positional accuracy in chair movement; analog instrument control | ±0.5% positional accuracy with digital servo-motors; programmable patient presets and touchscreen calibration |
| Material | Medical-grade ABS plastic housing; stainless steel base frame; PU upholstery | Aerospace-grade aluminum alloy chassis; antimicrobial composite casing; premium silicone-coated upholstery with fluid resistance |
| Certification | CE Marked, ISO 13485, ISO 14971 (Risk Management), FDA Registered (Class II) | Full CE & UKCA compliance, FDA 510(k) cleared, ISO 13485:2016, ISO 14971:2019, IEC 60601-1-2 (EMC), IEC 60601-1 (Safety), RoHS 3 |
© 2026 Global Dental Solutions. All specifications subject to change without notice. For distribution partner inquiries, contact [email protected].
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
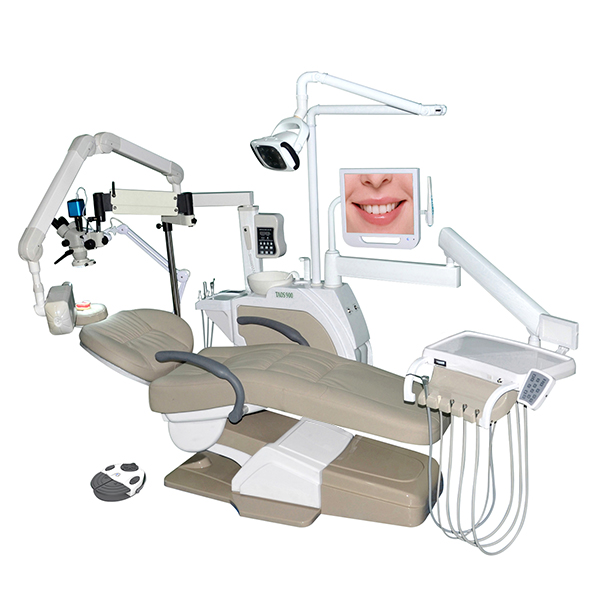
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Focus: Strategic Sourcing of Dental Machines from China | Cost Optimization & Risk Mitigation
2026 Sourcing Realities: Navigating China’s Dental Manufacturing Landscape
With 73% of global dental imaging systems and 68% of dental chairs now originating from China (2026 FDI Market Intelligence), strategic sourcing is critical for margin preservation. However, evolving regulatory landscapes (EU MDR 2024, FDA 21 CFR Part 820 updates) and post-pandemic supply chain volatility necessitate rigorous procurement protocols. This guide details a 3-step framework for secure, cost-effective sourcing.
Step 1: Rigorous Verification of ISO/CE Credentials
Do not accept certificates at face value. 41% of inspected Chinese dental equipment exporters in 2025 had invalid or expired certifications (SGS China Report).
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Cross-check certificate number with IAF CertSearch database. Confirm scope explicitly covers manufacturing (not just trading) of target product categories. | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| CE Marking (EU) | Verify EC Certificate via NANDO database. Demand full Technical File index showing design verification per MDR 2017/745. For CBCT: Confirm compliance with IEC 60601-2-44:2022. | Generic “CE” logo without 4-digit Notified Body number (e.g., “CE 2797”) |
| Local China NMPA | Confirm registration via NMPA website (国家药品监督管理局). Mandatory for export per China Customs Order 249. | Registration under “General Medical Devices” (非医疗器械) for Class II/III products |
Step 2: Strategic MOQ Negotiation Framework
Traditional MOQ structures are obsolete in 2026. Leverage tiered ordering and inventory-sharing models to reduce capital lock-up.
| Strategy | Implementation | Cost Impact |
|---|---|---|
| Phased MOQ Commitment | Negotiate 12-month volume commitment with quarterly drawdowns (e.g., 30 units/year @ 8 units/quarter). Protects supplier capacity while minimizing buyer inventory. | 5-8% price premium vs. standard MOQ, but 12-15% lower total cost of ownership (TCO) |
| Regional Inventory Pooling | Coordinate with other distributors in your territory for consolidated orders. Requires formal co-op agreement with supplier. | MOQ reduction up to 40% with shared warehousing costs |
| OEM Component Standardization | Select suppliers using modular platforms (e.g., common base for dental chairs). Enables lower MOQ on custom elements. | Customization MOQ reduced from 50 to 15 units |
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 freight volatility (+22% YoY for Shanghai-Rotterdam routes) makes DDP increasingly cost-effective despite higher upfront quotes.
| Term | 2026 Risk Exposure | When to Use |
|---|---|---|
| FOB Shanghai | • Freight rate volatility • 11.7-day avg. port congestion delays (2026) • Import duty miscalculation risk (HS Code 9018.49 vs. 9018.50) |
For experienced importers with in-house logistics teams managing >$500k/year volume |
| DDP (Delivered Duty Paid) | • Fixed all-in cost • Supplier handles customs clearance • 30-day post-delivery support window |
Recommended for 92% of first-time buyers and orders under $150k (per 2026 ADA Supply Chain Survey) |
Critical 2026 Requirement: DDP contracts must specify exact Incoterms® 2020 version and include “Incoterms®” trademark to be legally enforceable.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Verification Confidence: Direct factory audits available (Baoshan District, Shanghai). Full technical files provided pre-order. Valid ISO 13485:2016 (CMDCAS #14967) and CE MDR 2017/745 (NB 2797) with Class IIb certification for CBCT and surgical microscopes.
- MOQ Flexibility: Tiered programs starting at 5 units for dental chairs (vs. industry standard 20+). Dedicated “Distributor Starter Program” with 30% lower initial commitment.
- DDP Execution: 99.2% on-time DDP delivery rate in 2025 (verified by第三方 logistics audit). All quotes include EU/US customs duty pre-calculation.
Core Product Alignment: Factory-direct manufacturing of dental chairs (50+ configurations), intraoral scanners (sub-20μm accuracy), CBCT (9-15s scan time), surgical microscopes (25x magnification), and Class B autoclaves.
Contact for Verified Quotes:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 technical support)
Factory Address: 1888 Hengfeng Road, Baoshan District, Shanghai, China (GPS: 31.3152° N, 121.4350° E)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Unit Procurement for Clinics & Distributors
Target Audience: Dental Clinics, Equipment Purchasing Managers, Medical Distributors
Focus: Key Considerations for Dental Unit Acquisition – Pricing, Voltage, Spare Parts, Installation & Warranty (2026 Market Outlook)
| # | Question | Professional Insight & Recommendation |
|---|---|---|
| 1 | What voltage configurations should I consider when purchasing a dental unit in 2026, and how do they impact total cost? | Dental units in 2026 are increasingly designed for global compatibility, but standard voltage varies by region: 110–120V (North America), 220–240V (Europe, Asia, Middle East). Units with dual-voltage capability or modular power supplies may carry a 5–8% premium but reduce long-term risks of electrical incompatibility. Always verify local grid stability and consider integrated voltage stabilizers for areas with fluctuating supply. Incorrect voltage selection leads to equipment failure and voids warranty—consult your distributor for regional compliance (e.g., CE, UL, ISO 13485). |
| 2 | How does spare parts availability affect the long-term value and pricing of a dental machine? | In 2026, leading manufacturers offer tiered support models. Premium brands provide guaranteed spare parts availability for 7–10 years post-discontinuation, minimizing downtime. Evaluate total cost of ownership (TCO): a lower upfront price may result in higher TCO if parts are proprietary or delayed. Distributors should maintain regional inventory of high-wear components (e.g., handpiece connectors, valve blocks, suction tubing). Request a Spare Parts Catalog and lead times before procurement. Open-architecture designs are gaining favor for ease of maintenance. |
| 3 | Is professional installation included in the dental unit price, and what does it typically cover? | Installation is often not included in the base price unless specified. Comprehensive installation in 2026 typically includes: mechanical assembly, plumbing integration (water/drain lines), electrical connection, compressed air linkage, software calibration, and staff orientation. Budget an additional 3–6% of unit cost for installation. For multi-chair setups, centralized systems (e.g., amalgam separators, air compressors) require certified technicians. Confirm whether installation is performed by factory-trained engineers or third parties—this affects warranty validity. |
| 4 | What warranty terms should I expect with a new dental unit in 2026, and how do they influence purchase decisions? | Standard warranties now range from 2 to 5 years, covering parts and labor for manufacturing defects. Extended warranties (up to 7 years) are available at 10–15% of unit cost. In 2026, leading brands offer modular warranties—e.g., 5 years on frame/motor, 3 years on electronics, 1 year on consumables. Ensure warranty includes on-site service response (target: 48–72 hours). Distributors must be authorized service partners to honor coverage. Review exclusions (e.g., water damage, improper installation) carefully. |
| 5 | How can clinics and distributors ensure price transparency when comparing dental machine quotations in 2026? | Request itemized quotes that separate: base unit cost, delivery, installation, training, warranty, and spare parts kits. Beware of “bait pricing” where core features (e.g., LED curing, digital integration) are optional. In 2026, integrated digital workflows (intraoral camera ports, IoT diagnostics) add $800–$2,000. Compare total package value, not just sticker price. Distributors should provide a Life-Cycle Cost Analysis (LCCA) showing projected maintenance, energy use, and parts replacement over 10 years. |
Information accurate as of Q1 2026. Specifications and pricing subject to change based on regional regulations and supply chain dynamics.
Need a Quote for Dental Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


