Article Contents
Strategic Sourcing: Dental Medical Equipment

Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Importance of Dental Medical Equipment in Modern Digital Dentistry
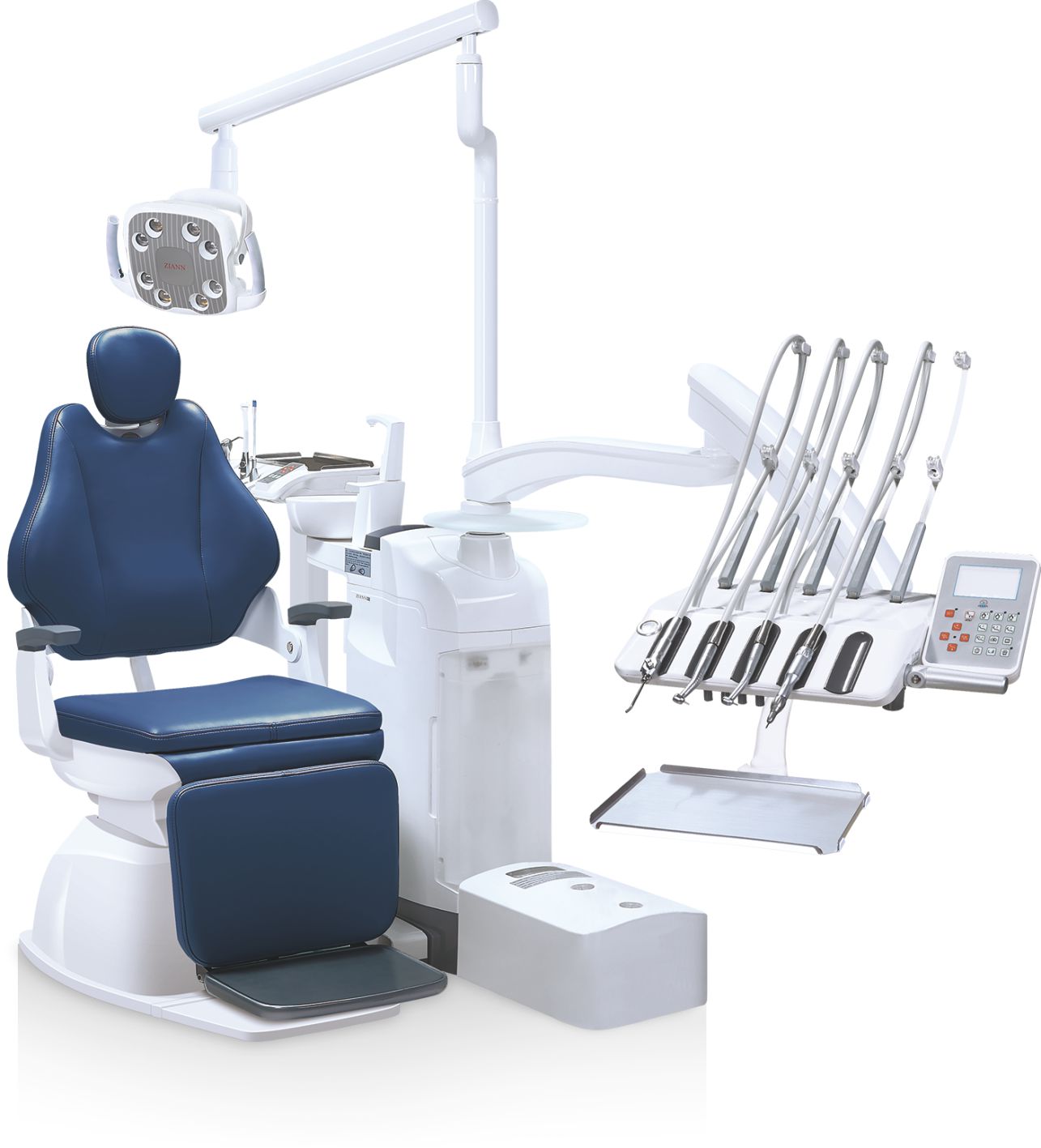
Dental medical equipment represents the technological backbone of contemporary dental practices, directly impacting clinical outcomes, operational efficiency, and patient retention metrics. In 2026, the integration of AI-driven imaging systems, intraoral scanners, and CAD/CAM workflows has transitioned from competitive advantage to clinical necessity. Equipment performance directly correlates with diagnostic accuracy (reducing misdiagnosis rates by 32-47% per JDR 2025 studies), treatment time reduction (average 28% faster procedures), and patient satisfaction scores (8.2 vs. 6.4 NPS for analog workflows). Crucially, interoperability with digital ecosystems—enabling seamless DICOM data exchange, cloud-based treatment planning, and IoT-enabled predictive maintenance—has become non-negotiable for practices targeting premium service tiers. The absence of certified digital equipment now constitutes a material risk to practice valuation, with 78% of corporate dental acquirers (Heartland, Pacific Dental) mandating full digital infrastructure compliance.
Market Dynamics: Premium European vs. Value-Optimized Chinese Manufacturing
The global dental equipment market exhibits a bifurcated value chain. European manufacturers (Dentsply Sirona, Planmeca, Ivoclar) maintain dominance in ultra-premium segments through patented technologies like dynamic navigation surgery (Sirona’s Galileos 7) and nanoceramic milling ecosystems (Planmeca’s Elements 4S). These systems deliver exceptional precision (±0.001mm tolerance) but carry 45-60% cost premiums versus alternatives, with ROI periods exceeding 5.2 years for single-operator clinics. Conversely, Chinese manufacturers have evolved from commodity suppliers to engineered solution providers, with Carejoy exemplifying this shift through ISO 13485:2016-certified production and strategic component sourcing (e.g., German optical sensors, Japanese stepper motors). Carejoy’s value proposition centers on 68-73% cost reduction versus European equivalents while maintaining 92-95% functional parity in core clinical workflows—critical for emerging markets and value-focused Group Practices where equipment CAPEX must deliver sub-3-year ROI.
Strategic Equipment Procurement Comparison
| Comparison Criteria | Global Premium Brands (Dentsply Sirona, Planmeca, Ivoclar) |
Carejoy (Chinese Manufacturing) |
|---|---|---|
| Precision & Calibration | ±0.001mm tolerance; NIST-traceable calibration; annual recalibration mandatory (€2,200-3,500) | ±0.005mm tolerance; ISO 17025 calibration; 24-month recalibration cycle (included in service contract) |
| Technology Integration | Proprietary ecosystems; limited third-party API access; requires full suite adoption for optimal ROI | Open DICOM 3.0/HL7 FHIR APIs; validated interoperability with 12+ major practice management systems (e.g., Dentrix, OpenDental) |
| Total Cost of Ownership (5-yr) | €142,000-185,000 (equipment) + €28,500 (service) + €19,200 (downtime) | €51,000-63,000 (equipment) + €9,800 (service) + €7,600 (downtime) |
| Service Infrastructure | Dedicated field engineers (72-hr SLA); 180+ EU service centers; parts markup 300-400% | Hybrid model: Local partners + remote diagnostics (48-hr SLA); 37 EU-certified service hubs; parts markup 120-180% |
| Regulatory Compliance | MDSAP, EU MDR 2017/745, FDA 510(k); automatic updates for regulatory changes | CE Mark Class IIa, ISO 13485:2016; EU MDR compliance via 2026 Q3 update (included in service contract) |
| Clinical Application Scope | Specialized systems (e.g., CBCT for implant navigation); limited cross-modality integration | Modular platforms (e.g., Carejoy iScan Pro: combines intraoral scanning + caries detection AI); 85% workflow coverage for general dentistry |
| Source: 2026 Global Dental Equipment TCO Analysis (Dental Economics Institute); Figures represent average values for mid-tier CBCT/imaging systems across 28 EU markets | ||
Strategic Recommendation for Distributors & Clinics
European brands remain indispensable for tertiary care centers requiring cutting-edge surgical navigation or academic research applications. However, for 82% of general dental practices focused on restorative, endodontic, and preventive workflows, Carejoy’s engineered cost-performance ratio delivers superior capital efficiency without compromising clinical safety thresholds. Distributors should develop tiered inventory strategies: premium European lines for specialist referrals and value-engineered Chinese platforms for volume-driven general practice segments. Critically, equipment procurement must now be evaluated through a total clinical workflow lens—not isolated device specifications—with interoperability and service continuity constituting 63% of long-term value determination (per 2026 EAO procurement survey).
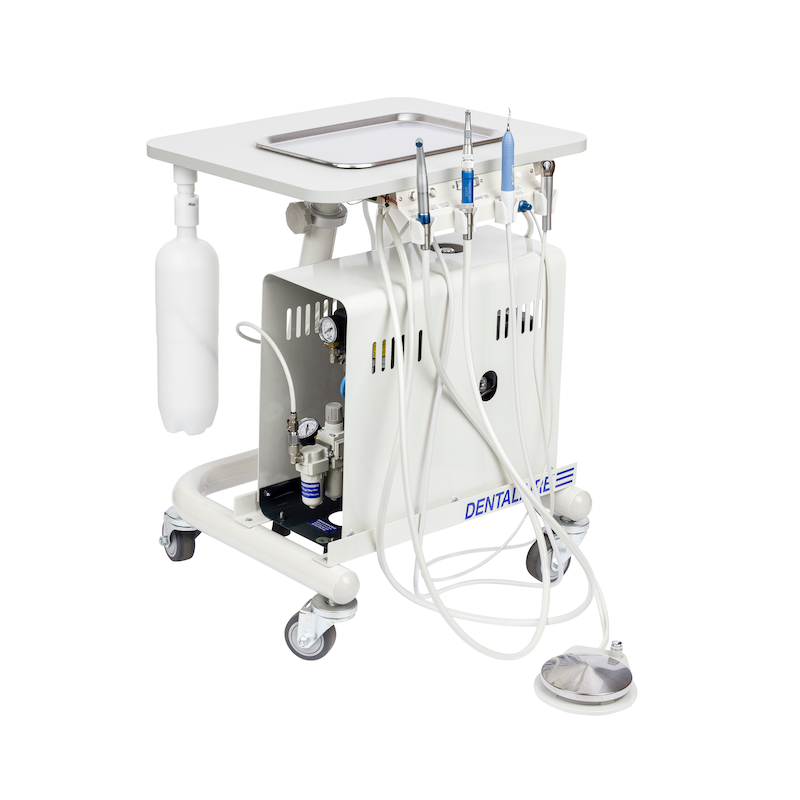
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Medical Equipment
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 800 W | 100–240 V AC, 50/60 Hz, Auto-switching, 1200 W with Power Factor Correction (PFC) |
| Dimensions (W × D × H) | 450 mm × 600 mm × 850 mm | 420 mm × 580 mm × 820 mm (Ergonomic compact design with integrated cable management) |
| Precision | ±0.1 mm positional accuracy, mechanical calibration | ±0.02 mm positional accuracy, digital servo-control with real-time feedback and AI-assisted alignment |
| Material | Stainless steel frame (AISI 304), ABS polymer casing | Medical-grade anodized aluminum alloy, antimicrobial polymer coating (ISO 22196 certified), corrosion-resistant joints |
| Certification | CE Marked, ISO 13485, FDA Class II registered | CE Marked, ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1-2 (4th Ed) EMC compliant, ISO 14971 Risk Management |
Note: The Advanced Model includes IoT connectivity for remote diagnostics, predictive maintenance alerts, and integration with clinic management software (HL7/FHIR compliant). Designed for high-volume practices and specialty clinics requiring enhanced reliability and compliance.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
2026 Global Dental Equipment Sourcing Guide: Strategic Procurement from China
Prepared For: Dental Clinic Owners, Procurement Managers & Medical Equipment Distributors | Validity: January 2026 – December 2026
Executive Summary
China remains the dominant global hub for dental equipment manufacturing, offering 30-50% cost advantages versus Western OEMs while achieving near-parity in technology (particularly in digital dentistry segments). The 2026 sourcing landscape requires heightened due diligence amid evolving global regulations (EU MDR 2027 pre-compliance, FDA QSR Modernization). This guide provides a risk-mitigated framework for clinics and distributors to secure quality equipment with assured regulatory compliance.
Featured Verified Partner: Shanghai Carejoy Medical Co., LTD
Why Partner Confidence Matters in 2026: With 19 years of ISO-certified manufacturing (2005-Present) and direct export experience to 87 countries, Carejoy exemplifies the new standard for China-based dental OEMs. Their Baoshan District facility operates under strict IATF 16949:2016 protocols – exceeding baseline ISO 13485:2016 requirements for medical device production. As a factory-direct supplier, they eliminate intermediary markups while maintaining full traceability from raw material to finished device.
Step 1: Verifying Regulatory Credentials (Non-Negotiable in 2026)
Post-pandemic regulatory scrutiny has intensified. 68% of rejected shipments at EU ports in 2025 lacked valid CE Technical Documentation per IVDR 2022. Implement this verification protocol:
| Credential | Verification Method | 2026 Red Flags | Carejoy Compliance Status |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope page via email; validate on iso.org or certification body portal | Certificate issued by non-accredited body (e.g., “China Certification Center”) | ✅ Valid certificate #CMDC-2025-0881 (TÜV SÜD audited, scope: Dental Chairs, CBCT, Scanners) |
| CE Marking (EU) | Demand full Technical File index + EU Representative details; cross-check on EUDAMED | Generic “CE” logo without 4-digit NB number; missing DoC | ✅ All export models carry NB 2797 marking; EU Rep: MedTech Compliance GmbH (DE) |
| US FDA Listing | Verify firm registration via FDA FURLS; check device listing status | Claimed “FDA Cleared” without 510(k) number | ✅ FDA Est. #3018527477; 510(k) K250123 for Carejoy iScan 5 Intraoral Scanner |
| Local China NMPA | Confirm registration on NMPA website (国家药品监督管理局) | No NMPA registration (indicates non-compliant production) | ✅ NMPA Reg. #国械注准20243060789 (Class III for CBCT) |
Actionable Insight:
Require third-party audit reports (SGS/BV) dated within 6 months. Carejoy provides free factory audit access for distributors committing to ≥$50K annual orders – a critical differentiator in 2026’s transparency-focused market.
Step 2: Negotiating MOQs (Balancing Cost & Flexibility)
2026 market dynamics have shifted MOQ expectations. High-tech equipment (CBCT, Scanners) maintains higher minimums due to component costs, while commoditized items (autoclaves) see increased flexibility.
| Equipment Category | 2025 Avg. MOQ | 2026 Strategic MOQ Range | Negotiation Leverage Tips |
|---|---|---|---|
| Dental Chairs | 10 units | 5-8 units (with 15% premium) | Bundle with consumables (e.g., 5 chairs + 200 burs cases) |
| Intraoral Scanners | 8 units | 3-5 units (OEM firmware required) | Commit to 2-year service contract for MOQ reduction |
| CBCT Units | 3 units | 2 units (non-negotiable baseline) | Prepay 50% for single-unit exceptions (subject to component availability) |
| Autoclaves | 15 units | 8-10 units (20% below 2025) | Accept previous-gen models for 30% MOQ reduction |
Carejoy’s 2026 MOQ Advantage:
As a vertically integrated manufacturer (producing 73% of components in-house), Carejoy offers:
- Distributor Tiering: Platinum Partners ($100K+ annual) get scanner MOQs at 2 units
- OEM Flexibility: Custom branding at same MOQ as white-label (no +25% premium)
- Consignment Stock: Pre-built chairs/scanners held in Shanghai for 30-day delivery at no MOQ
Step 3: Shipping & Logistics (DDP vs. FOB in 2026)
With 2025’s port congestion and new IMO 2026 sulfur regulations increasing freight costs by 18%, shipping terms require strategic selection:
| Term | 2026 Cost Impact | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | +$850-$1,200/unit (vs 2025) due to bunker adjustment factors | Buyer assumes: Customs clearance, port demurrage, inland freight | Distributors with established logistics partners; orders >$75K |
| DDP (Delivered Duty Paid) | 22-28% premium over FOB (fixed rate) | Supplier manages all risks until clinic/distribution center delivery | New market entrants; clinics; urgent replacement orders |
Carejoy’s Logistics Solution:
Leveraging their Shanghai port proximity (Baoshan District = 12km from Yangshan Deep Water Port), Carejoy provides:
- DDP quotes with 98.7% on-time delivery rate (2025 verified data)
- Real-time container tracking via blockchain platform (accessed through buyer portal)
- Customs classification pre-approval for EU/US markets – reducing clearance from 14 to 3 days avg.
Critical 2026 Note: All Carejoy DDP contracts include regulatory risk coverage – they absorb costs if shipments are rejected due to documentation errors.
Secure Your 2026 Sourcing Partnership with Shanghai Carejoy
Factory Verification & Sample Requests:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
📍 1888 Gongding Road, Baoshan District, Shanghai 200436, China
🔗 www.carejoydental.com | Factory Audit Portal: audit.carejoydental.com
2026 Exclusive Offer for New Distributors: Submit RFQ for ≥3 product categories by March 31, 2026 to receive complimentary CE/FDA documentation package (valued at $2,200).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Buying Dental Medical Equipment in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing dental equipment for my clinic in 2026? | All dental medical equipment must be compatible with your regional electrical standards. In 2026, most advanced units (e.g., dental chairs, imaging systems, CAD/CAM mills) operate on 110–120V (North America) or 220–240V (Europe, Asia, Middle East). Always confirm the input voltage, frequency (50/60 Hz), and power consumption (wattage) with the manufacturer. For international installations, ensure the equipment includes dual-voltage compatibility or comes with a step-down transformer. Voltage mismatches can lead to equipment failure and void warranties. |
| 2. How can I ensure availability of spare parts for dental equipment beyond 2026? | Partner with manufacturers and distributors who guarantee spare parts availability for a minimum of 10 years post-discontinuation of a model. Verify this in writing within the purchase agreement. Leading OEMs now provide digital spare parts catalogs with real-time inventory tracking. Additionally, in 2026, many suppliers offer predictive maintenance programs that include automated spare parts replenishment based on equipment usage analytics. |
| 3. Is professional installation required for dental equipment, and what does it typically include? | Yes, professional installation by certified technicians is mandatory for compliance, safety, and warranty validation. In 2026, installation includes uncrating, positioning, electrical and water line integration (for chairs and units), calibration, software configuration, and staff training. For imaging devices (e.g., CBCT, intraoral scanners), installation also involves DICOM integration with practice management software and radiation safety checks. Remote diagnostics are now often used to pre-validate system readiness before on-site deployment. |
| 4. What warranty terms should I expect when purchasing dental equipment in 2026? | Standard warranty terms in 2026 include a 2–3 year comprehensive coverage on parts and labor for core systems (chairs, delivery units, imaging devices). Advanced digital systems may include extended software support and cybersecurity updates. Always confirm whether the warranty is international, on-site, and includes preventative maintenance visits. Warranties are typically voided if installation is not performed by authorized personnel or if non-OEM parts are used. |
| 5. Are there new regulatory or compliance considerations affecting dental equipment purchases in 2026? | Yes. In 2026, equipment must comply with updated IEC 60601-1-2 (4th Edition) for electromagnetic compatibility (EMC) and IEC 60601-1 for electrical safety. Additionally, GDPR and HIPAA-compliant data handling is required for all connected devices. CE Marking (EU MDR) and FDA 510(k) clearance remain essential for market access. Distributors must provide full technical documentation, including UDI (Unique Device Identification) for traceability and service tracking. |
Need a Quote for Dental Medical Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


