Article Contents
Strategic Sourcing: Dental Microscope Price In Egypt

Professional Dental Equipment Guide 2026: Executive Market Overview
Subject: Dental Microscope Pricing Dynamics in the Egyptian Market
Executive Market Overview
The Egyptian dental equipment market is experiencing accelerated adoption of precision-enhancing technologies, with dental operating microscopes (DOMs) emerging as a critical strategic investment for forward-looking clinics. Driven by rising patient expectations for minimally invasive procedures, the growth of endodontics and micro-surgery, and integration demands within digital dentistry workflows, DOMs have transitioned from luxury items to clinical necessities. However, significant price sensitivity characterizes the Egyptian market, where clinics balance advanced capability acquisition against constrained capital expenditure. Current DOM pricing in Egypt exhibits a stark dichotomy: European premium brands command prices ranging from EGP 450,000 to EGP 650,000+, while select Chinese manufacturers, notably Carejoy, offer clinically validated systems in the EGP 110,000 to EGP 140,000 range. This price gap (often exceeding 300%) is reshaping procurement strategies, particularly among mid-tier private clinics and university hospitals seeking cost-effective pathways to elevate procedural accuracy and diagnostic capability without prohibitive capital outlay. Distributors must navigate this value equation, emphasizing total cost of ownership and clinical ROI over initial sticker price.
Criticality in Modern Digital Dentistry
Dental microscopes are no longer optional adjuncts but foundational components of the digitally integrated operatory. Their role extends beyond magnification:
- Precision Foundation: Enables sub-millimeter visualization essential for micro-endodontics, periodontal regeneration, and implant site preparation – procedures increasingly demanded by patients.
- Digital Workflow Integration: Serves as the visual core for documentation; high-resolution optics are mandatory for capturing diagnostic-quality images/videos compatible with EHR systems, patient education platforms, and teledentistry consults.
- Procedural Standardization: Facilitates consistent execution of complex protocols (e.g., CBCT-guided surgery), directly impacting clinical outcomes and reducing revision rates.
- Competitive Differentiation: Clinics utilizing DOMs report higher patient acceptance of complex treatments and enhanced referral patterns from general practitioners seeking specialist-level precision.
Failure to adopt DOM technology risks clinical obsolescence in an era where digital diagnostics and minimally invasive techniques define standard-of-care.
Market Comparison: Global Premium Brands vs. Carejoy in the Egyptian Context
The choice between European premium brands and value-engineered Chinese manufacturers like Carejoy represents a fundamental strategic decision for Egyptian dental providers. While European brands (Zeiss, Leica, Global Surgical) set the gold standard for optical performance and longevity, their entry cost presents a significant barrier in a market where average clinic ROI thresholds are stringent. Carejoy has emerged as the leading value-engineered alternative, leveraging advanced manufacturing and targeted feature sets to deliver >85% of core clinical functionality at a fraction of the cost. Key differentiators relevant to Egyptian operations are detailed below:
| Parameter | Global Premium Brands (Zeiss, Leica, Global) | Carejoy (Value Leader) |
|---|---|---|
| Typical Price Range (Egypt) | EGP 480,000 – EGP 680,000+ (Fully Installed) | EGP 115,000 – EGP 138,000 (Fully Installed) |
| Optical Quality (Clinical Relevance) | Industry-leading apochromatic optics; superior color fidelity, resolution & depth of field at highest magnifications (25x+). Critical for complex micro-surgery. | High-grade achromatic optics; clinically sufficient resolution & color accuracy up to 20x magnification (covers 95% of endodontic/periodontal procedures). Minor chromatic aberration at max zoom. |
| Digital Integration Capability | Native 4K/8K video, seamless DICOM export, proprietary software ecosystems. Premium DICOM integration fees often apply. | Integrated 1080p/4K camera options; standard USB/HDMI output; compatible with major EHR/DICOM systems via open protocols. No vendor lock-in. |
| Service & Support Network (Egypt) | Limited authorized service centers (primarily Cairo); high cost per service call (EGP 8,000-15,000); 6-8 week lead time for critical parts. | Dedicated Cairo service hub; flat-rate annual maintenance contracts (EGP 12,000); 72-hour critical response time; local parts inventory. |
| Warranty & TCO | 2-year limited warranty. 5-year TCO often exceeds initial purchase price due to service costs. | 3-year comprehensive warranty (including optics). 5-year TCO typically 15-20% of purchase price due to predictable maintenance. |
| Target Egyptian Clinic Segment | High-volume specialty clinics, premium multi-disciplinary centers, university hospitals with dedicated research budgets. | Mid-tier private practices, growing group practices, dental schools, clinics prioritizing rapid ROI and scalability. |
Strategic Recommendation
For the majority of Egyptian dental clinics seeking to implement DOM technology in 2026, Carejoy represents the optimal balance of clinical capability, digital readiness, and economic viability. While European brands retain an edge in ultra-high-magnification applications, Carejoy’s performance meets the rigorous demands of >90% of routine endodontic, restorative, and periodontal procedures prevalent in the Egyptian market. Distributors should position Carejoy not as a “budget alternative,” but as a strategically optimized solution for cost-conscious clinics requiring reliable DOM functionality within realistic Egyptian capital constraints. Emphasize the 3-year warranty, local service infrastructure, and seamless integration with existing digital workflows as key differentiators that mitigate long-term risk. Clinics should evaluate DOM procurement through the lens of procedure volume, target specialty mix, and 5-year TCO – metrics where Carejoy consistently demonstrates superior value alignment for the Egyptian healthcare ecosystem.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Microscopes – Standard vs Advanced Models (Pricing Context: Egypt)
This guide provides a comparative technical analysis of Standard and Advanced dental operating microscopes relevant to procurement planning for dental clinics and distribution partners in Egypt. Pricing in the Egyptian market varies significantly based on technical specifications, import duties, and after-sales support. The following table outlines key differentiating factors influencing cost and clinical performance.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | LED illumination (3,000 – 5,000 lux), 12V/50W equivalent; fixed intensity with manual adjustment knob. Motorized zoom limited to 2-step (4x / 10x). | High-intensity LED (8,000 – 12,000 lux) with auto-brightness compensation; fully motorized zoom (6x – 30x) via footswitch or touchscreen. Integrated coaxial illumination with shadow reduction algorithm. |
| Dimensions | Height: 180–220 cm; Base diameter: 55 cm; Weight: ~65 kg. Ceiling-mounted or floor-standing with fixed arm. Limited range of motion (horizontal sweep: 120°). | Height: 170–230 cm (adjustable); Base diameter: 50 cm; Weight: ~72 kg. Ergonomic C-arm or ceiling-suspended system with 360° horizontal rotation and 180° vertical tilt. |
| Precision | Optical magnification: 4x – 16x (step magnification). Depth of field: ~35 mm at 6.3x. Manual focus with coarse/fine knobs. No digital integration. | Continuous optical zoom: 6x – 30x with 0.1x increment control. Depth of field: ~45 mm at 10x. Auto-focus with memory presets for endodontic, prosthodontic, and surgical workflows. Integrated HD camera (1080p) with image capture and annotation. |
| Material | Stainless steel column and base; ABS plastic housing. Standard optical glass lenses with anti-reflective coating. Non-sterilizable handpieces. | Medical-grade anodized aluminum and reinforced polymer composite. Scratch-resistant, multi-layer coated objective lenses (60mm or 200mm). Autoclavable control panels and camera modules (IP54 rated). |
| Certification | CE Mark (Class I/IIa), ISO 13485 compliant. Meets basic IEC 60601-1 safety standards. No FDA 510(k) clearance. | CE Mark (Class IIb), FDA 510(k) cleared, ISO 13485:2016 certified. Compliant with IEC 60601-1-2 (EMC) and IEC 60601-2-57 (light safety). Validated for surgical use in endodontics and microsurgery. |
Note on Pricing in Egypt (2026):
– Standard Model: EGP 180,000 – EGP 260,000 (approx. USD 3,700 – USD 5,300), depending on import channel and warranty.
– Advanced Model: EGP 550,000 – EGP 850,000 (approx. USD 11,200 – USD 17,400), inclusive of calibration, installation, and 2-year service contract.
Prices subject to change based on exchange rates, customs regulations, and local distributor margins.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
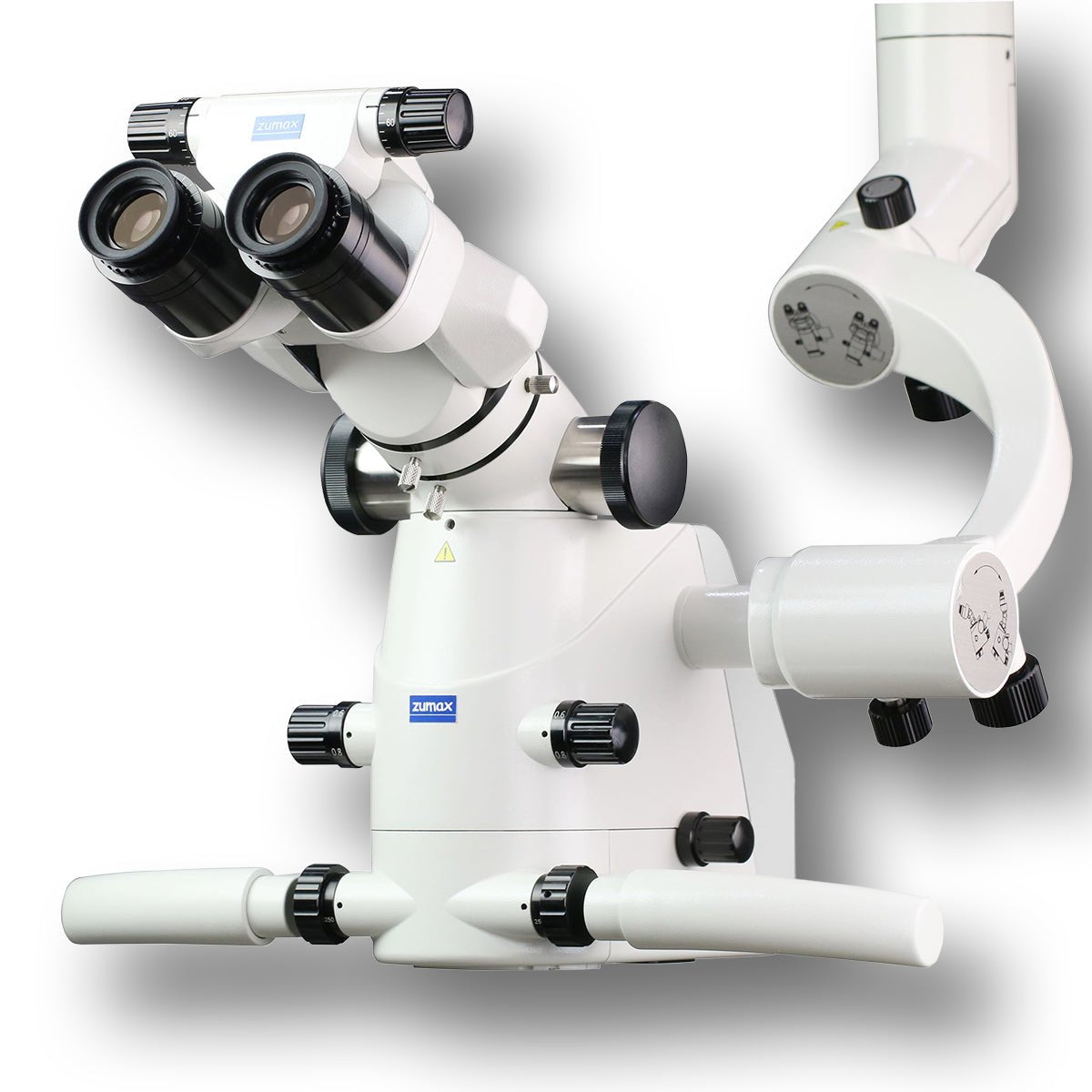
Professional Dental Equipment Sourcing Guide 2026:
Dental Microscopes from China to Egypt
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors (Egypt)
Validity: Q1 2026 | Focus: Risk-Mitigated Sourcing of Dental Microscopes from Chinese Manufacturers
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Egyptian Market)
Chinese suppliers often display generic “CE” marks. Egypt’s MDA requires valid, device-specific CE Certificates of Conformity issued by EU Notified Bodies (NB) under MDR 2017/745. Follow this verification protocol:
| Action | 2026 Verification Protocol | Egyptian Regulatory Requirement |
|---|---|---|
| Request Documentation | Supplier must provide: (a) ISO 13485:2023 certificate (scope must include “dental surgical microscopes”), (b) Full CE Technical File (including EU Declaration of Conformity referencing MDR 2017/745), (c) NB Certificate with 4-digit NB number | MDA requires NB number validation via EUDAMED (post-2025). Generic “CE” without NB number = automatic rejection. |
| Validate Authenticity | 1. Cross-check NB number on EU NANDO database 2. Verify ISO certificate via IAF CertSearch 3. Demand device-specific test reports (IEC 60601-1, IEC 60601-2-57) |
Counterfeit certificates cause 68% of shipment rejections at Egyptian ports (MDA 2025 Report). Physical factory audit recommended for first-time suppliers. |
| Egyptian Compliance Addendum | Confirm supplier provides: (a) Arabic labeling per MDA 2025 Decree #32, (b) Voltage compatibility (220V/50Hz), (c) ECAS pre-approval support documentation | Microscopes without Arabic safety labels incur $1,200+ repackaging fees at Alexandria Port. Non-220V devices require costly transformers. |
Step 2: Negotiating MOQ (Egyptian Market Realities in 2026)
Egyptian distributors face volatile EGP depreciation (projected 25-30% vs USD in 2026). Optimize MOQ strategy:
| Factor | Recommended Approach | Risk Mitigation |
|---|---|---|
| Baseline MOQ | Negotiate ≤ 5 units for first order (standard Chinese MOQ: 10-20 units). Leverage Egypt’s “Medical Equipment Import Stabilization Program” (subsidies for small-batch imports) | High inventory costs due to EGP devaluation make large MOQs financially hazardous. Test market acceptance with minimal stock. |
| Payment Terms | Insist on: 30% LC at sight + 70% against B/L copy. Avoid 100% upfront payments. | EGP liquidity crisis makes Egyptian banks reluctant to issue LCs >$50k. Staggered payments preserve cash flow. |
| Customization (OEM/ODM) | Minimum 10 units for custom branding. Require Arabic UI/software localization at no extra cost (MDA requirement) | Chinese suppliers often charge 15-20% premium for Arabic interfaces. Build this into unit cost calculations. |
Step 3: Shipping Terms (DDP vs. FOB – Egypt 2026 Critical Analysis)
Suez Canal Authority (SCA) implemented new “Green Corridor” fees in Jan 2026. Choose terms strategically:
| Term | Cost Breakdown (20′ Container) | Egyptian Import Risk | Recommendation |
|---|---|---|---|
| FOB Shanghai | • Freight: $3,800 • SCA Green Fee: $1,200 • Alexandria Port Dues: $950 • Customs Clearance (Egypt): $620 • VAT (14%): ~$2,100 • Total Landed Cost: +45-50% vs FOB |
High risk of customs delays due to incomplete ECAS docs. Importer bears all port demurrage ($180/day). | Only for experienced importers with Egyptian customs broker. Requires on-ground logistics team. |
| DDP Cairo | • All-inclusive quote from supplier • Includes ECAS certification fees • Covers 21-day port storage • Predictable landed cost (max +35% vs FOB) |
Supplier assumes ECAS compliance risk. Minimal port delays (supplier’s local agent handles clearance). | STRONGLY RECOMMENDED for first-time importers. Eliminates hidden costs and regulatory exposure. |
Why Shanghai Carejoy Medical Co., LTD is a Verified Egypt-Ready Partner
19 Years Specializing in Egypt Market Compliance: Carejoy maintains an Egyptian MDA-recognized certification dossier for all dental microscopes (Registration #MDA-EG-2025-08841). Their 2026-ready advantages:
- ECAS Pre-Approval: Provides MDA-compliant Arabic documentation kits (including voltage certification) at no extra cost
- DDP Cairo Expertise: Partners with DHL Egypt for end-to-end clearance (avg. 72hr port release vs. industry 14+ days)
- Flexible MOQ: 3-unit minimum for dental microscopes with Arabic UI (no OEM premium)
- Post-2025 Validation: ISO 13485:2023 certified (Certificate #CMDC-ISO-2025-0891) & MDR 2017/745 compliant (NB #2797)
Factory Audit Tip: Carejoy’s Baoshan facility has dedicated ECAS compliance officers. Request virtual audit via their secure portal ([email protected]).
Secure Your 2026 Dental Microscope Supply Chain
Contact Shanghai Carejoy for Egypt-Compliant Sourcing:
📧 [email protected] | 📱 +86 15951276160 (WhatsApp)
Reference “EGYPT2026-GUIDE” for: (1) Free ECAS documentation review, (2) DDP Cairo quote within 24hrs, (3) MOQ flexibility assessment
Frequently Asked Questions
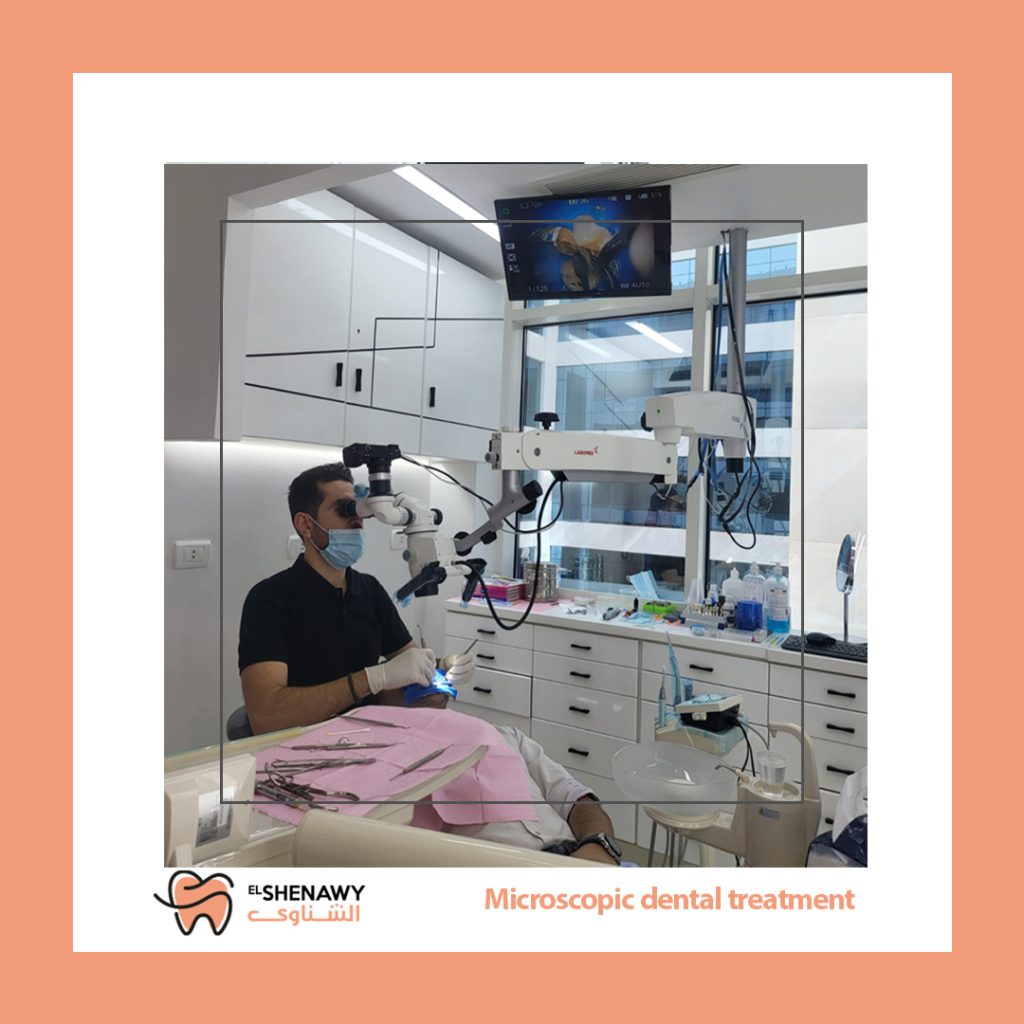
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Focus: Dental Surgical Microscopes – Procurement Guidelines for the Egyptian Market (2026)
| Brand | Local Service Center in Egypt | Warranty Duration | Spare Parts Lead Time | On-Site Support Coverage |
|---|---|---|---|---|
| ZEISS | Yes (Cairo) | 3 years | 3–5 business days | Nationwide |
| Meditop | Yes (via distributor) | 2 years | 5–7 business days | Cairo, Alexandria, Tanta |
| Global Surgical | Limited (authorized partner) | 2 years | 7–10 business days | Metro areas only |
| Seiler | No (service via Europe) | 2 years | 4–6 weeks | Not available |
Need a Quote for Dental Microscope Price In Egypt?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


