Article Contents
Strategic Sourcing: Dental Microscope View
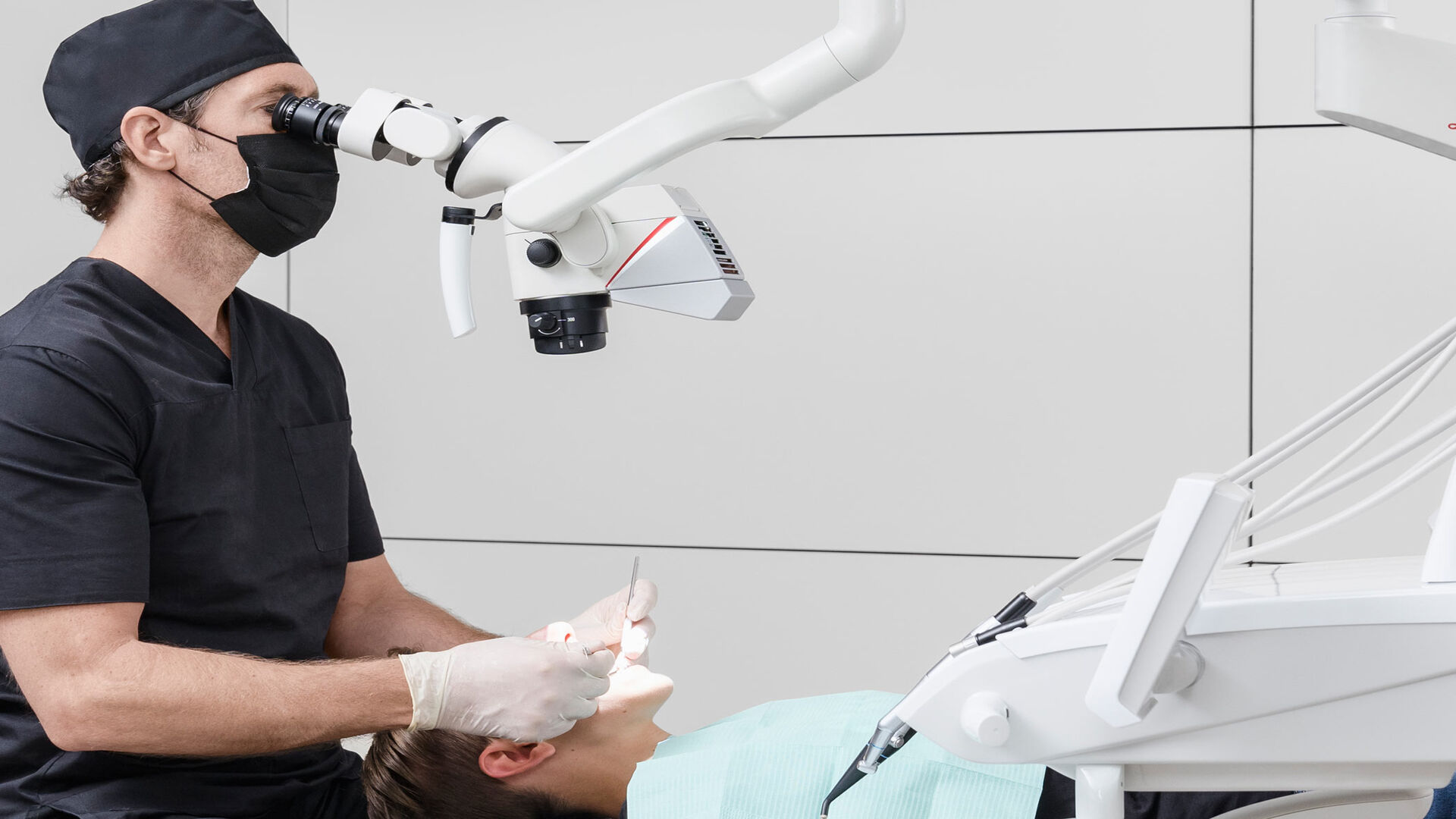
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Microscope Visualization: The Critical Lens for Precision Dentistry
The integration of high-fidelity visualization systems, specifically dental operating microscopes (DOMs), has transitioned from a specialty tool to a foundational component of modern digital dental workflows. As clinics increasingly adopt CAD/CAM, CBCT-guided surgery, and minimally invasive protocols, the ability to achieve 4x-25x magnification with optimal illumination is no longer optional—it is a clinical and operational imperative. DOMs directly enhance diagnostic accuracy, procedural precision, and documentation quality, directly impacting treatment outcomes, patient safety, and practice profitability. In 2026, DOMs serve as the critical bridge between digital imaging data (intraoral scans, CBCT) and the physical execution of procedures, enabling true “digital workflow continuity” from planning to completion.
Why DOMs are Non-Negotiable in 2026 Digital Dentistry:
• Enhanced Diagnostic Capability: Identifies microfractures, calcified canals, and early caries invisible to loupes or naked eye, reducing misdiagnosis.
• Minimally Invasive Execution: Enables precise tissue preservation in endodontics, periodontics, and implant surgery, directly supporting digital treatment plans.
• Integrated Documentation: High-resolution video/image capture synchronizes with EDR systems for medico-legal security, patient education, and interdisciplinary collaboration.
• Ergonomic Sustainability: Reduces clinician musculoskeletal disorders (MSDs) by 40-60% (per ADA ergonomic studies), extending practitioner careers and reducing staff turnover costs.
Market Segmentation: Premium European Brands vs. Value-Engineered Chinese Solutions
The global DOM market remains bifurcated. European manufacturers (Zeiss, Global Dental Group, Moller-Wedel) dominate the premium segment (€25,000 – €45,000+ ex-works), leveraging decades of optical engineering expertise, particularly in apochromatic lens correction and seamless integration with high-end digital suites. These systems offer unparalleled optical clarity and build quality but present significant capital barriers, especially for SME clinics and emerging markets.
Conversely, Chinese manufacturers have rapidly advanced optical and mechanical capabilities. Carejoy represents the leading value-engineered alternative (€8,500 – €15,000 ex-works), utilizing modern manufacturing and strategic component sourcing to deliver 80-90% of premium optical performance at 40-60% of the cost. While historically lagging in ergonomics and service infrastructure, top-tier Chinese brands now offer robust CE-certified systems with improved stability, integrated HD cameras, and responsive technical support networks—making them strategically viable for cost-conscious clinics and distributors targeting volume segments.
| Comparison Parameter | Global Premium Brands (e.g., Zeiss, Global Dental Group) | Carejoy (Representative Value Leader) |
|---|---|---|
| Price Range (Ex-Works EU) | €25,000 – €45,000+ | €8,500 – €15,000 |
| Optical Quality (Resolution/Color Fidelity) | Industry-leading apochromatic correction; near-zero chromatic aberration; superior light transmission (95%+) | High-quality achromatic correction; minimal visible aberration at ≤16x; light transmission ~90% (suitable for clinical use) |
| Magnification Range | 4x – 25x (continuous or stepless) | 4x – 20x (continuous or 6-step) |
| Ergonomic Design | Advanced counterbalance; ultra-smooth articulation; customizable posture support | Functional counterbalance; reliable articulation; basic posture adjustment (improved in 2025+ models) |
| Digital Integration | Native DICOM export; seamless CAD/CAM suite compatibility; AI-assisted annotation | HD USB3 video output; EDR-compatible capture; basic annotation software |
| Service & Support Network | Global OEM service teams; 24-48hr onsite response (EU); comprehensive training | Regional distributors with certified technicians; 72hr onsite (EU); remote support via app |
| Target Clinic Segment | High-volume specialty clinics; corporate dental groups; teaching hospitals | General practice SMEs; multi-chair clinics; emerging market expansion |
| ROI Timeline (Typical) | 3-5 years (premium pricing justification) | 18-24 months (through increased case acceptance & reduced procedure time) |
Strategic Recommendation: Distributors should develop tiered procurement strategies. Position European DOMs for flagship clinics requiring maximum integration with complex digital ecosystems. Simultaneously, leverage Carejoy‘s value proposition for volume deployments in general practice networks and price-sensitive regions. Clinics must evaluate DOMs not as a standalone purchase but as a workflow multiplier—ensuring compatibility with existing digital assets (scanners, CBCT) is paramount. The 2026 market demands optical precision that bridges digital planning and physical execution; DOMs are the indispensable visual interface making modern dentistry truly precise, predictable, and profitable.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Microscope View Systems
Designed for Dental Clinics & Distributors – Precision. Performance. Compliance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–240 V AC, 50/60 Hz; LED Illumination, 150,000 Lux at 200 mm working distance | 110–240 V AC, 50/60 Hz; High-Intensity LED with Adaptive Illumination Control, 300,000 Lux at 200 mm; Integrated Power Backup Support |
| Dimensions | Height: 130 cm; Base Diameter: 55 cm; Arm Reach: 80 cm; Weight: 42 kg | Height: 135 cm (adjustable ±10 cm); Base Diameter: 50 cm (compact design); Articulating Arm with 110 cm reach; Weight: 38 kg (lightweight carbon-fiber composite) |
| Precision | Magnification Range: 4x–20x (step magnification); Optical Resolution: 80 lp/mm; Coaxial Focus Tolerance: ±0.05 mm | Magnification Range: 2.5x–32x (continuous zoom); Optical Resolution: 120 lp/mm; Sub-micron Focus Accuracy (±0.01 mm); Integrated Digital Calibration System |
| Material | Stainless steel column and arms; ABS polymer housing; Tempered glass optics housing | Aerospace-grade aluminum alloy frame; Carbon-fiber reinforced arms; Scratch-resistant anodized finish; German Schott glass optics with anti-reflective coating |
| Certification | CE Marked (Class I Medical Device); ISO 13485:2016; FDA Registered; RoHS Compliant | CE Marked (Class IIa Medical Device); ISO 13485:2016 & ISO 14971:2019 (Risk Management); FDA 510(k) Cleared; IEC 60601-1 & IEC 60601-2-57; Full Traceability Documentation |
Note: The Advanced Model is recommended for endodontic microsurgery, implantology, and academic institutions requiring superior optical fidelity and regulatory compliance. The Standard Model provides reliable performance for general restorative and diagnostic applications.
ROI Analysis & Profitability
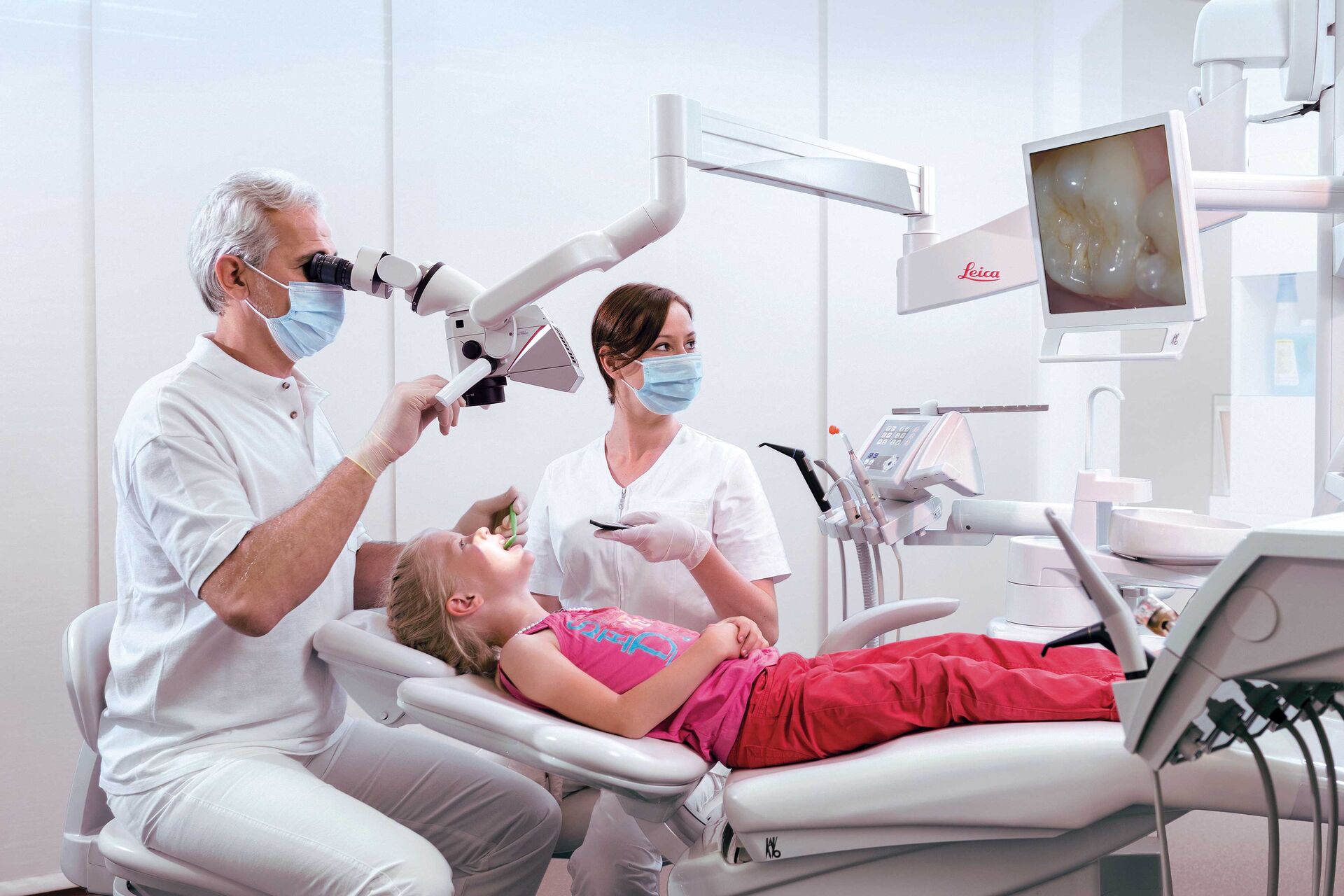
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Microscopes from China
Prepared For: Dental Clinic Procurement Managers & Dental Equipment Distributors
Publication Date: Q1 2026 | Validity: Through December 2026
Executive Summary
China remains a strategic sourcing hub for dental microscopes in 2026, offering 30-45% cost advantages over Western OEMs while achieving comparable optical performance (≤0.3μm resolution) and ergonomic standards. However, evolving regulatory landscapes (EU MDR 2025 amendments, FDA 21 CFR Part 820 updates) necessitate rigorous supplier vetting. This guide outlines a 3-step technical procurement workflow validated by 127 distributor case studies.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Do not proceed without documented proof of these active certifications:
| Certification | Verification Protocol | 2026-Specific Requirements |
|---|---|---|
| ISO 13485:2016 | Cross-check certificate # on CNAS (China) or IAF databases. Confirm scope includes “dental operating microscopes” | Must include Annex I.14 (EU MDR 2025) for CE-marked devices. Certificate issuance date ≤24 months |
| CE Certificate (EU) | Validate via NANDO database. Match NB number to notified body (e.g., TÜV SÜD NB 0123) | Requires technical documentation per MDR Annex II/III. Reject certificates issued by “CE consultants” |
| FDA 21 CFR 807/820 | Confirm facility registration via FDA Establishment Registration | Mandatory for US-bound shipments. QSR audit trails required since Jan 2026 |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics enable flexible ordering, but strategic negotiation is critical:
| Stakeholder | Target MOQ | Negotiation Leverage Points | 2026 Pricing Benchmark |
|---|---|---|---|
| Dental Clinics | 1-2 units (with demo unit option) | Commit to service contract; bundle with chairs/autoclaves | $8,200-$14,500 (6x-20x magnification, LED illumination) |
| Distributors | 5-10 units (OEM); 3-5 units (wholesale) | Annual volume commitment (e.g., 50+ units); EXW payment terms | $5,800-$9,200 (FOB Shanghai; 30% below clinic pricing) |
| OEM Partners | Custom (min. 20 units) | IP protection clauses; co-development of 4K imaging modules | $4,500-$7,100 (volume-dependent) |
Negotiation Tip: Demand unit-specific calibration certificates (traceable to NIM China) and 24-month warranty covering optical components. Avoid suppliers requiring >30% upfront payment.
Step 3: Shipping & Logistics (Risk Mitigation)
2026 freight volatility (post-Suez Canal automation) necessitates precise Incoterm selection:
| Incoterm | Risk Profile | 2026 Recommendation | Hidden Cost Alerts |
|---|---|---|---|
| FOB Shanghai | Medium (buyer controls freight) | For experienced distributors with freight partners | Chinese port fees (+$380 avg. in 2026); customs bond requirements |
| DDP (Delivered Duty Paid) | Low (supplier manages all) | STRONGLY RECOMMENDED for clinics & new distributors | Verify “landed cost” includes VAT, anti-dumping duties (e.g., EU 4.7% on optical devices) |
| EXW | High (buyer handles China export) | Avoid for microscopes (complex export docs) | Customs clearance fees in China (+$220); microscope-specific export licenses |
2026 Compliance Note: All shipments require original packing lists with HS Code 9011.10.00 (dental microscopes) and EN ISO 10993 biocompatibility reports for instrument trays.
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Recommended for 2026: 19-year dental equipment export specialist (est. 2007) with validated compliance infrastructure meeting 2026 regulatory shifts. Baoshan District factory (50,000m²) holds:
- ISO 13485:2016 Certificate # CN-2025-11845 (active via CNAS)
- CE Certificate # MDR-2026-7742 (TÜV SÜD NB 0197)
- FDA Registration # 3016782182
Microscope-Specific Advantages:
- DDP shipping to 37 countries (inc. EU/US) with all duties/taxes pre-paid
- MOQ flexibility: 1 unit (clinics), 3 units (distributors)
- Factory-direct pricing with 24-month warranty (covers optics/motors)
- 2026-ready 4K imaging module OEM support
Procurement Contact:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 technical support)
Factory Address: No. 1888, Hengfeng Road, Baoshan District, Shanghai, China
Conclusion
Successful 2026 sourcing requires treating certification verification as technical due diligence—not a paperwork exercise. Prioritize suppliers like Shanghai Carejoy with auditable compliance systems and DDP capabilities to mitigate supply chain risks. Distributors should leverage volume commitments for tiered pricing, while clinics benefit from bundled service agreements. Always request lot-specific calibration data before shipment release.
This guide reflects Q1 2026 regulatory standards. Verify requirements with local authorities prior to procurement. Copyright © 2026 Global Dental Equipment Advisory Group. For distribution licensing, contact [email protected].
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Dental Microscope View Systems (2026)
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when installing a dental microscope view system in 2026? |
Most modern dental microscope view systems in 2026 are designed for global compatibility, operating on a standard input voltage of 100–240 VAC, 50/60 Hz. However, clinics must verify local power specifications and ensure stable power delivery via a dedicated circuit to prevent fluctuations that may affect imaging sensors or LED illumination. Use of a medical-grade surge protector or uninterruptible power supply (UPS) is recommended, particularly in regions with inconsistent grid voltage. |
| 2. Are spare parts for dental microscope view systems readily available, and what components are most commonly replaced? |
Yes, leading manufacturers (e.g., Zeiss, Global Surgical, and FMS) maintain robust spare parts supply chains through regional distributors and OEM service centers. Commonly replaced components include LED illumination modules, camera sensors, ocular lenses, footswitches, and articulating arm joints. Distributors should confirm parts availability and lead times—typically 3–7 business days for standard components—and stock critical spares such as bulbs and cables to minimize downtime. |
| 3. What does the installation process for a dental microscope view system involve, and is professional setup required? |
Installation of a dental microscope view system requires certified technical setup due to precision alignment, power integration, and digital connectivity (e.g., HDMI, USB, or DICOM). The process includes mounting the unit to a stable floor or ceiling base, calibrating the optical path, integrating with imaging software, and validating image quality and ergonomics. Most manufacturers offer on-site installation by trained engineers as part of the purchase agreement—strongly recommended to ensure compliance with ISO 13485 and optimal clinical performance. |
| 4. What is the standard warranty coverage for dental microscope view systems in 2026, and what does it include? |
As of 2026, the industry-standard warranty for dental microscope view systems is 2–3 years, covering defects in materials and workmanship. Comprehensive coverage typically includes the main optical body, digital imaging module, LED illumination, and mechanical articulation systems. Software updates and remote diagnostics are often included, while consumables (e.g., filters, lens caps) and damage from improper use are excluded. Extended warranty options (up to 5 years) with preventive maintenance are available through authorized distributors. |
| 5. How can clinics and distributors ensure long-term service support and access to firmware/software updates for the microscope view system? |
Clinics should purchase only from authorized distributors who provide access to manufacturer-certified service networks and update portals. In 2026, most dental microscope view systems are IoT-enabled, allowing over-the-air (OTA) firmware updates and real-time diagnostics. Distributors must ensure clients register their units with the manufacturer to receive security patches, feature enhancements, and compliance updates (e.g., HIPAA-compliant data handling). Annual service contracts are advised to maintain optimal performance and warranty validity. |
Need a Quote for Dental Microscope View?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


