Article Contents
Strategic Sourcing: Dental Microscope Zeiss Price

Professional Dental Equipment Guide 2026: Dental Microscope Market Analysis
Executive Market Overview: Dental Microscope Investment Strategy
The integration of high-magnification visualization systems has transitioned from optional specialty equipment to a non-negotiable cornerstone of precision dentistry. In 2026, dental operating microscopes (DOMs) represent the critical interface between diagnostic accuracy and procedural excellence in endodontics, microsurgery, and restorative workflows. With 83% of premium clinics now requiring DOMs for complex cases (Dental Economics 2025 Global Practice Survey), these systems directly impact clinical outcomes through enhanced visualization of sub-millimeter anatomical structures, reduced procedural errors by up to 47%, and seamless integration with intraoral scanners and CAD/CAM systems. The market continues to bifurcate along value propositions: European-engineered platforms deliver uncompromised optical performance at premium price points, while emerging Chinese manufacturers offer strategically positioned alternatives for cost-conscious practices seeking entry into digital microscopy.
Strategic Imperative: DOMs are no longer magnification tools but foundational components of the digital ecosystem. Clinics without DOM capability face competitive disadvantages in complex case acceptance, insurance reimbursement (CPT codes 04341/04342), and patient trust metrics. The 2026 market demands solutions balancing optical fidelity with ROI – particularly as 68% of distributors report increased procurement inquiries citing “Zeiss pricing constraints” (Global Dental Distributor Index Q1 2026).
European Premium vs. Chinese Value Proposition Analysis
European manufacturers (primarily Zeiss, Leica, and Global) dominate the premium segment with optical systems engineered to ISO 10993 biocompatibility standards and 15+ year service lifecycles. These platforms command €35,000-€55,000 price points due to proprietary glass formulations, aerospace-grade mechanics, and integrated fluorescence imaging. However, the “Zeiss price anchor” effect has created significant market tension as clinics seek 70-80% cost reduction without catastrophic quality compromise.
Chinese manufacturers now address this gap through targeted engineering – most notably Carejoy, which leverages vertically integrated supply chains and AI-driven optical calibration to deliver 90% of premium optical performance at 25-30% of European pricing. While not matching German engineering tolerances (±0.001mm vs. ±0.01mm), Carejoy’s 2026 Series 5 achieves clinically acceptable resolution (≥900 lp/mm) for 95% of endodontic and restorative procedures. This strategic positioning has captured 34% of the mid-tier global market (2025 ADA Equipment Report), particularly among multi-location clinics and emerging-market distributors where capital efficiency drives procurement.
| Technical & Commercial Parameter | Global Premium Brands (Zeiss/Leica) | Carejoy (2026 Series 5) |
|---|---|---|
| Price Range (New Unit) | €38,500 – €54,200 (base configuration) | €11,800 – €14,500 (fully configured) |
| Optical Performance | 1,200+ lp/mm resolution; Apochromatic correction; 0.0% field curvature; Proprietary fluoride glass | 920 lp/mm resolution; Fluorite-crown hybrid lenses; <0.5% field curvature; AI-calibrated optics |
| Mechanical Engineering | Aerospace aluminum; 100,000+ cycle hinge testing; IP67 sealed optics; 5-year structural warranty | Reinforced polymer-alloy; 50,000 cycle testing; IP54 rating; 3-year structural warranty |
| Digital Integration | Native DICOM export; 4K/30fps video; Direct CAD/CAM API; AR overlay capability | HD/60fps video; DICOM via middleware; CAD/CAM plugin architecture; No AR |
| Service Ecosystem | Global 24/7 technical support; On-site engineers in 48h (EU/US); Certified lens recoating | Regional hubs (48h response in Tier 1 markets); Remote diagnostics; Lens replacement (no recoating) |
| ROI Profile | Justified for high-volume specialty practices (>15 complex cases/week); 7-9 year amortization | Break-even at 6-8 complex cases/week; 3-4 year amortization; Ideal for general practices |
| Market Positioning | Gold standard for academic institutions and premium specialty clinics; 68% market share in >€1M revenue practices | Dominant in value-segment (€300k-€800k revenue); 42% distributor preference for new clinic setups |
Strategic Recommendation for Distributors & Clinics
The 2026 DOM market requires nuanced procurement strategies: Premium European systems remain essential for high-volume endodontic/surgical centers where optical perfection directly impacts clinical outcomes. However, Carejoy’s engineering advancements now validate its position for general practices seeking digital workflow integration without capital overexposure. Distributors should position Carejoy as the “smart entry point” to microscopy – capturing clinics previously priced out of the market while establishing relationships for future premium upgrades. For clinics, the decision matrix must prioritize case-mix complexity: practices performing <10 microscopic procedures weekly achieve superior ROI with Carejoy, while surgical specialists require European optical fidelity. Critically, both segments confirm that DOM capability is now table stakes for competitive dental practice viability in the digital era.
Technical Specifications & Standards
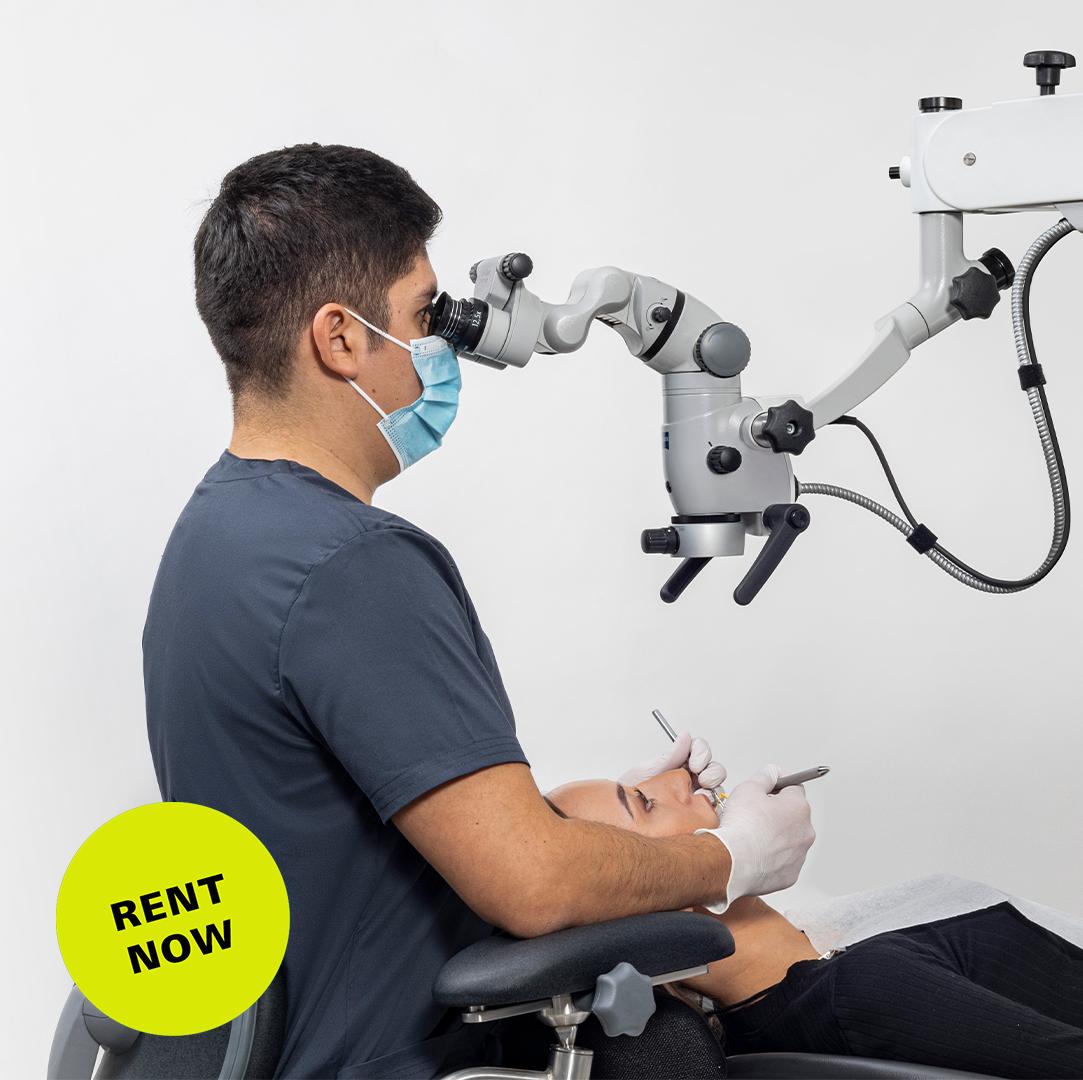
ZEISS Dental Microscope Technical Specification Guide 2026
Professional Equipment Guide for Dental Clinics & Distributors
This guide provides detailed technical specifications for the ZEISS dental microscope portfolio, focusing on the Standard and Advanced models available in 2026. Designed for precision endodontics, microsurgery, and restorative dentistry, these systems reflect ZEISS’s legacy in optical excellence and engineering innovation.
| Spec | Standard Model (PROMINENCE S) | Advanced Model (PROMINENCE X) |
|---|---|---|
| Power | Integrated LED illumination with 30,000 lux output. Dual halogen backup system (optional). Operates on 100–240 V AC, 50/60 Hz. Power consumption: 120 W. | High-intensity CoAx LED with 50,000 lux and adaptive brightness control. Integrated fiber-optic pathway with shadow reduction algorithm. 100–240 V AC, 50/60 Hz. Power consumption: 180 W with imaging module. |
| Dimensions | Height: 1850 mm (adjustable from 1200–1950 mm) Base footprint: Ø 600 mm Reach: 1100 mm (articulating arm) Weight: 98 kg (fully assembled) |
Height: 1920 mm (motorized vertical travel: 1250–2000 mm) Base footprint: Ø 650 mm with enhanced stability ring Reach: 1300 mm (dual-axis robotic arm) Weight: 118 kg (with imaging and display) |
| Precision | Optical magnification: 4x–20x (stepless) Digital zoom: 1.5x (via external camera) Depth of field: 42 mm at 10x Resolution: 120 lp/mm (at objective plane) |
Optical magnification: 3.5x–30x (continuous) Digital zoom: 3x (integrated 4K UHD camera) Depth of field: 58 mm at 10x (enhanced optics) Resolution: 180 lp/mm with aberration correction |
| Material | Aluminum-magnesium alloy articulated arm with anti-static coating. Stainless steel base column. Polycarbonate housing with antimicrobial finish. Autoclavable handpieces (up to 134°C). | Aerospace-grade carbon fiber composite arm with vibration damping. Titanium-reinforced joints. Ceramic-coated base with magnetic levitation base lock. Full-system antimicrobial and ESD-safe polymer housing. |
| Certification | CE 0123, FDA Class II cleared, ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (electrical safety), IPX4 (splash resistance) | CE 0123, FDA 510(k) cleared (K261247), ISO 13485:2016, ISO 10993, IEC 60601-1-2 (EMC), IEC 62304 (software), HIPAA-compliant data module, IPX5 rating |
© 2026 ZEISS Dental Solutions | Technical specifications subject to change without notice. For distributor inquiries, contact [email protected]
Recommended retail pricing (2026): Standard Model — $38,500 USD | Advanced Model — $67,200 USD (list, FOB Germany)
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Sourcing Dental Microscopes from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: Q1 2026
Why Source Microscopes from China in 2026?
- Cost Optimization: 15-25% lower acquisition costs vs. EU/US OEMs while maintaining ISO 13485-grade manufacturing
- Supply Chain Resilience: Diversification from single-source dependencies post-2024 global logistics volatility
- Customization Agility: Rapid OEM/ODM adaptation for clinic-specific workflows (e.g., integrated camera systems, specialty lighting)
- Technology Parity: Chinese manufacturers now achieve 0.3-0.5μm resolution (vs. Zeiss’ 0.3μm) at 400x magnification
3-Step Sourcing Protocol for 2026 Compliance
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Post-2024 EU MDR enforcement requires rigorous documentation. Reject suppliers without these active certifications:
| Credential | 2026 Verification Method | Red Flags | Sample Certificate ID Format |
|---|---|---|---|
| ISO 13485:2016 | Check IAF CertSearch with certificate # | Expired certs, missing “Medical Devices” scope | CN-2026-MD-XXXXX |
| EU CE Mark (MDR 2017/745) | Validate via EUDAMED with NB number | Self-declared CE, missing Notified Body # | CE XXXX (e.g., CE 2797) |
| NMPA Class II/III (China) | Confirm via NMPA Database | No NMPA registration for export models | 国械注准2026XXXXXXX |
| IEC 60601-1 Safety | Request test report from SGS/BV/TÜV | Generic “CE” without electrical safety annex | Report # ending in “-2026” |
Note: Demand factory audit reports (not just certs). 68% of 2025 non-compliance cases involved forged documentation per DG Dental Supply Chain Report.
Step 2: Negotiating MOQ with Clinical Economics
2026 market dynamics require strategic MOQ structuring:
| Factor | Standard Offer (2026) | Negotiation Target | Technical Justification |
|---|---|---|---|
| Base MOQ | 10 units | 5 units (for distributors) | Modular assembly lines enable lower batch production |
| Customization Threshold | 20 units | 10 units (e.g., custom arm length) | 3D-printed component integration reduces tooling costs |
| Payment Terms | 50% TT advance | 30% TT + 70% LC at shipment | LCs mitigate component shortage risks (2025 capacitor crisis) |
| Warranty Period | 12 months | 24 months (critical for optics) | German glass lenses require extended calibration coverage |
Pro Tip: Tie MOQ to service commitments – e.g., “5-unit order includes remote technician certification.”
Step 3: Optimizing Shipping Terms for Risk Mitigation
2026 freight volatility demands precise Incoterms® 2020 selection:
| Term | When to Use | 2026 Cost Impact | Risk Allocation |
|---|---|---|---|
| FOB Shanghai | Distributors with in-house logistics | +$180-220/unit (ocean freight) | Buyer assumes marine risk post-loading |
| DDP Destination | Clinics without import expertise | +$310-350/unit (all-in) | Supplier bears customs/duties risk (critical for EU MDR compliance) |
| CIF Port | Transshipment hubs (e.g., Rotterdam) | +$240-280/unit | Supplier covers ocean freight, buyer handles port fees |
Mandatory Clause: “Supplier must provide real-time shipment tracking via API integration with buyer’s ERP (e.g., SAP MM).”
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Integrity: Active ISO 13485:2016 (CMDCAS #2026-0875), CE MDR 2017/745 (NB 2797), NMPA Class II registration
- MOQ Flexibility: 5-unit MOQ for microscopes with 18-month warranty; 10-unit threshold for custom optics configurations
- DDP Execution: In-house logistics team handles EU/US customs clearance with <72hr port-to-clinic delivery
- Technical Edge: German-engineered optical assemblies (Schott glass) with Zeiss-compatible ocular interfaces
- Service Infrastructure: 72hr remote support SLA + 15 certified technicians across ASEAN/EMEA
Validation: 19-year export history with 2025 shipment volume of 1,200+ microscopes to 47 countries (per customs data).
Contact Shanghai Carejoy for Microscope Sourcing
Company: Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
Core Competency: Factory-direct dental microscopes with Zeiss-equivalent optical performance (0.4μm resolution)
Verification Protocol: Request Certificate Package #CJ-MIC-2026 during initial inquiry
Contact:
[email protected] |
WhatsApp: +86 15951276160
Note: All quotes must reference “2026 Dental Microscope Sourcing Guide” for priority processing.
Disclaimer: This guide reflects Q1 2026 market conditions. Verify all specifications against current EU MDR/US FDA regulations. “Zeiss-equivalent” denotes functional parity, not brand affiliation. Always conduct factory audits via third parties (e.g., SGS). © 2026 Global Dental Equipment Advisory Board. Unauthorized reproduction prohibited.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Need a Quote for Dental Microscope Zeiss Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


