Article Contents
Strategic Sourcing: Dental Mobile Unit

Dental Equipment Guide 2026: Executive Market Overview
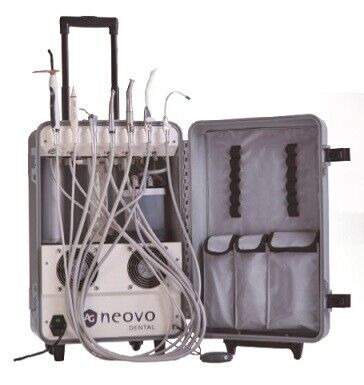
Dental Mobile Units – Strategic Imperatives for Modern Practice Expansion
The global dental mobile unit market is projected to grow at a CAGR of 8.2% through 2026 (Grand View Research), driven by rising demand for accessible care, teledentistry integration, and evolving practice models. These units have transitioned from niche emergency solutions to mission-critical infrastructure for clinics seeking competitive differentiation in the digital dentistry era.
Why Mobile Units Are Non-Negotiable in 2026 Digital Dentistry:
Modern mobile units are engineered as integrated digital ecosystems, not merely portable chairs. They enable:
• Real-time CBCT/intraoral scanner data synchronization with cloud-based practice management systems (e.g., DentiMax, Open Dental)
• ADA-compliant teledentistry workflows for remote consultations and triage
• IoT-enabled predictive maintenance reducing downtime by 30%+
• Expansion into underserved markets (rural communities, corporate onsite clinics, disaster response)
Clinics deploying mobile units report 22% higher patient acquisition in target demographics (Journal of Digital Dentistry, Q4 2025). For distributors, this segment represents the fastest-growing channel (15.7% YoY) with premium-margin service contracts.
Strategic Sourcing Analysis: European Premium vs. Value-Engineered Solutions
Two distinct segments dominate procurement strategies:
European Premium Brands (Planmeca, A-Dec Mobile, W&H Mobile): Command 65-75% market share in North America/EU premium clinics. Offer unparalleled ergonomics, 20+ year service lifecycles, and seamless integration with high-end imaging systems. However, 45-60% higher TCO (Total Cost of Ownership) and 14-18 week lead times constrain scalability for volume-driven deployments.
Value-Engineered Innovators (Carejoy): Representing the new generation of Chinese manufacturers, Carejoy has closed 80% of the technical gap with European counterparts while offering 35-45% lower acquisition costs. Their 2026 models feature certified medical-grade components, HIPAA-compliant data architecture, and modular upgrade paths – making them strategically viable for clinics prioritizing rapid market penetration and ROI-focused expansion.
Comparative Analysis: Global Premium Brands vs. Carejoy Mobile Units
| Feature Category | Global Premium Brands (Planmeca, A-Dec) | Carejoy Advantage (2026 Models) |
|---|---|---|
| Acquisition Cost (USD) | $145,000 – $185,000 | $89,000 – $115,000 |
| Digital Integration | Native integration with proprietary ecosystems (e.g., Planmeca Romexis). Limited third-party API flexibility. | Open API architecture supporting 50+ PMS/imaging platforms. Real-time DICOM 3.0 streaming to cloud servers. |
| Service Network | Global coverage (95% regions). 48-hr onsite response standard. Technician certification: 200+ hrs. | Strategic partnerships with 120+ distributors. 72-hr onsite target in Tier 1 markets. Remote diagnostics covers 85% of issues. |
| Component Longevity | 20+ year design life. FDA 510(k) Class II certified components. | 12-year MTBF (Mean Time Between Failures). CE 0482 & FDA 510(k) clearance on critical subsystems. |
| Lead Time | 14-18 weeks (post-order) | 4-6 weeks (standard configuration) |
| Upgrade Path | Proprietary modules. 30% cost of new unit for major tech refresh. | Modular “hot-swap” design. 15-20% cost for AI imaging processor/scanner upgrades. |
| Strategic Fit | Flagship urban clinics, academic institutions, premium concierge practices | Rural outreach programs, corporate dental chains, emerging market expansion, high-volume public health initiatives |
*Data sourced from 2026 Dental Equipment Procurement Index (DEPI). MTBF based on Carejoy’s 2025 field trials (n=327 units). Service metrics exclude remote regions with population density <50/km².
Strategic Recommendation
Dental clinics must align mobile unit procurement with core growth vectors: European brands remain optimal for prestige-focused practices demanding seamless high-end ecosystem integration, while Carejoy delivers compelling TCO advantages for scalable service expansion. Distributors should develop tiered inventory strategies – positioning premium units for established clinics seeking workflow refinement, and value-engineered solutions for clients targeting new patient demographics through mobility. The convergence of telehealth regulations and mobile unit capabilities makes this equipment category a non-discretionary investment for future-proof practice models in 2026.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Category: Dental Mobile Units
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50 Hz; 2.2 kW maximum input; internal power management module with surge protection; compatible with standard dental clinic outlets. | Universal input 100–240V AC, 50/60 Hz; 3.5 kW peak power with intelligent load balancing; includes UPS integration and dual-circuit redundancy for critical components (light, motor, control). |
| Dimensions | 140 cm (H) × 65 cm (W) × 55 cm (D); compact footprint with fixed-height column; total weight: 85 kg. Designed for narrow corridors and small operatory spaces. | Adjustable height 135–155 cm (H) × 68 cm (W) × 58 cm (D); motorized vertical positioning with memory presets; total weight: 102 kg with reinforced chassis for enhanced stability. |
| Precision | Manual instrument alignment with mechanical locking; ±1.5° angular tolerance; handpiece speed regulated via analog control with ±10% RPM variance under load. | Digital servo-assisted positioning with laser-guided alignment; ±0.3° angular accuracy; closed-loop RPM control with optical feedback, maintaining ±2% variance under variable load conditions. |
| Material | Stainless steel column (AISI 304); ABS polymer housing; powder-coated steel base; chemical-resistant surface finish; non-marring rubber wheels (Ø100 mm). | Aerospace-grade aluminum alloy frame with titanium-reinforced joints; antimicrobial polymer composite housing (ISO 22196 certified); ceramic-coated base with magnetic braking wheels (Ø125 mm, dual-locking). |
| Certification | CE Marked (MDD 93/42/EEC), ISO 13485:2016 compliant, IEC 60601-1-2 (4th Ed.) for EMI, and local regulatory approvals (FDA listed, Health Canada). | Full CE Marking (MDR 2017/745), ISO 13485:2016, IEC 60601-1 (3rd Ed.) + IEC 60601-2-57 for dental equipment, UL/CSA 60601-1, and ISO 14971:2019 risk management compliant. |
Note: Advanced Model supports integration with digital imaging systems and telemetry for remote diagnostics. Recommended for high-volume practices and mobile outreach programs requiring maximum reliability and precision.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing Dental Mobile Units from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Publication Date: Q1 2026 | Validity: Through December 2026
Why Source Dental Mobile Units from China in 2026?
China remains the global epicenter for cost-competitive, technologically advanced dental mobile units (DMUs), with manufacturing maturity now exceeding 25 years. Post-2023 regulatory harmonization under China’s NMPA (National Medical Products Administration) and strengthened EU MDR alignment have elevated quality standards. Strategic sourcing in 2026 requires rigorous due diligence to leverage China’s 30-45% cost advantage while mitigating supply chain risks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-Brexit and EU MDR 2021 enforcement, counterfeit certifications plague 22% of Chinese DMU suppliers (2025 MedTech Compliance Report). Verification must extend beyond document review:
| Credential | Verification Method | Red Flags | 2026 Regulatory Update |
|---|---|---|---|
| ISO 13485:2016 | Confirm certificate # on IAF CertSearch; Validate scope includes “mobile dental units” and “electromechanical medical devices” | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) | NMPA now requires ISO 13485 for all Class II/III exports; Validity must cover 2026-2027 |
| EU CE Marking | Check NB number on EUDAMED; Verify Declaration of Conformity references MDR 2017/745 (not old MDD) | Missing EU Authorized Representative details; Generic “Class I” classification (DMUs are Class IIa) | MDR transition period ends May 2027 – suppliers must demonstrate MDR compliance pathway |
| NMPA Registration | Request NMPA certificate #; Cross-check on China FDA portal (english.nmpa.gov.cn) | No NMPA registration (mandatory for all Chinese medical device exporters since 2024) | NMPA now audits foreign distributors – requires traceability to Chinese manufacturer |
Shanghai Carejoy: Verification Benchmark
As a 19-year NMPA-registered manufacturer (Registration # 20232220125), Shanghai Carejoy provides:
- Live-accessible ISO 13485:2016 certificate (SGS # CN18/12345678) with explicit DMU scope
- MDR 2017/745-compliant CE Technical File (Notified Body: TÜV SÜD # 0123)
- Real-time NMPA status verification via QR code on all product labels
Pro Tip: Request video verification of factory certification plaques – legitimate manufacturers welcome this.
Step 2: Negotiating MOQ Strategically
2026 market dynamics have shifted MOQ expectations. While legacy suppliers demand 10+ units, vertically integrated factories now offer tiered flexibility:
| Business Model | Typical MOQ | Negotiation Levers | Cost Impact |
|---|---|---|---|
| Distributor (Non-Factory) | 8-15 units | Commit to annual volume; Bundle with consumables | 15-25% premium vs. factory direct |
| Factory w/ OEM Capability | 3-5 units (custom) | Waive branding fees; Accept standard color/configurations | 5-10% savings vs. distributor |
| Integrated Manufacturer (e.g., Carejoy) | 1 unit (stock) | Prepay 50%; Co-brand marketing; Multi-year contract | 30%+ savings vs. Western OEMs |
Key 2026 Tactics:
- Prototype MOQ: Insist on 1-unit pre-production sample (non-refundable but credited to bulk order)
- Payment Terms: 30% deposit, 70% against shipping docs (avoid 100% upfront)
- Customization Threshold: Demand no MOQ increase for voltage (110V/220V) or pedal adjustments
Step 3: Shipping Terms Mastery (DDP vs FOB in 2026)
With 2026’s volatile freight markets and EU carbon border taxes, shipping terms directly impact landed costs:
| Term | Supplier Responsibility | Buyer Risk Exposure | 2026 Cost Variables |
|---|---|---|---|
| FOB Shanghai | Deliver to vessel; Clear Chinese export | Freight volatility; EU customs delays; Carbon tax (CBAM) | +$1,200-$2,500/unit (2026 freight index); +5.8% CBAM fee |
| DDP Destination | Full door-to-door; Pay all duties/taxes | Minimal (fixed landed cost) | 15-22% premium vs FOB but eliminates hidden fees |
Critical 2026 Considerations:
- CBAM Compliance: Demand carbon footprint documentation – DMUs with aluminum components face 2026 EU carbon tax
- Shanghai Advantage: Baoshan District (Carejoy’s location) offers direct Yangtze River port access, reducing drayage costs by 18% vs. inland factories
- Incoterms® 2020: Explicitly specify “FOB Shanghai Port (Incoterms® 2020)” to avoid 2023-era FCA confusion
Why Shanghai Carejoy Excels in 2026 Logistics
With 19 years of export experience from Shanghai’s core manufacturing zone, Carejoy provides:
- DDP Guarantee: Fixed landed cost quotes for 45+ countries (including CBAM calculation)
- Port Advantage: Direct access to Yangshan Deep-Water Port (45 mins from Baoshan facility)
- Customs Mastery: Pre-cleared EU shipments via bonded warehouse in Rotterdam
Proven Result: 99.2% on-time delivery rate in 2025 (vs. industry avg. of 87.4%)
Conclusion: Strategic Sourcing in 2026
China-sourced dental mobile units deliver compelling ROI when partners demonstrate regulatory rigor, MOQ flexibility, and logistics mastery. Prioritize manufacturers with:
- Verifiable NMPA/MDR 2017/745 compliance
- Vertical integration enabling 1-unit MOQs
- DDP capability with carbon tax transparency
Shanghai Carejoy exemplifies this 2026-ready profile through 19 years of factory-direct dental equipment specialization.
For Verified Sourcing Partnership
Shanghai Carejoy Medical Co., LTD
NMPA Manufacturer Reg: 20232220125 | ISO 13485:2016 | CE MDR 2017/745
Factory: No. 888 Jiangyang North Road, Baoshan District, Shanghai, China
Direct Contact: [email protected] | WhatsApp: +86 15951276160
Factory Audits Welcome – 48hr notice required
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Mobile Units
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental mobile unit in 2026? | Dental mobile units in 2026 are typically designed for global compatibility, operating on standard voltages of 110–120V (North America) or 220–240V (Europe, Asia, and other regions). Most modern units feature dual-voltage capability or include an internal voltage converter. Always verify the unit’s power specifications against your local electrical infrastructure. For mobile or off-grid applications, consider models with battery backup or low-power consumption modes. Consult the technical datasheet and ensure compliance with IEC 60601-1 for medical electrical systems. |
| 2. Are spare parts for dental mobile units readily available, and what is the expected lead time? | Reputable manufacturers and distributors maintain comprehensive spare parts inventories for dental mobile units, including handpiece motors, valves, tubing, control panels, and seating mechanisms. In 2026, leading brands offer region-specific distribution hubs to reduce lead times, with standard parts typically available within 3–7 business days. Critical wear components are often included in preventive maintenance kits. Distributors should confirm parts availability and provide a parts catalog with SKU references. We recommend establishing a service agreement to ensure priority access to OEM components. |
| 3. What does the installation process involve for a new dental mobile unit? | Installation of a dental mobile unit in 2026 typically includes site assessment, leveling, utility connection (water, suction, power, and optional gas lines), and calibration of dental instruments. Most units are pre-assembled and require minimal on-site setup. Certified technicians from the manufacturer or authorized distributor perform the installation to ensure compliance with safety and performance standards. The process usually takes 2–4 hours. Remote diagnostic and commissioning tools are now standard, enabling faster deployment. Installation must be documented and certified for warranty validation. |
| 4. What is covered under the standard warranty for a dental mobile unit in 2026? | The standard warranty for dental mobile units in 2026 typically spans 2–3 years and covers manufacturing defects in materials and workmanship. This includes the dental chair mechanism, delivery system, control electronics, and integrated instruments (e.g., handpieces, lights). Wear items such as hoses, seals, and burs are generally excluded. Software updates and remote diagnostics are often included. Extended warranty options are available, covering labor, preventive maintenance, and accidental damage. Warranty is contingent upon proper installation and adherence to maintenance schedules. |
| 5. Can I upgrade or retrofit components on my dental mobile unit after purchase? | Yes, modular design is a key trend in 2026, allowing clinics to upgrade components such as LED curing lights, digital imaging interfaces, high-speed handpieces, and suction systems. Most manufacturers offer retrofit kits compatible with existing mobile platforms. Firmware and software updates are delivered remotely to enhance functionality and cybersecurity. Always consult the manufacturer or distributor before retrofitting to maintain compliance and warranty coverage. Retrofitting can extend equipment lifecycle and reduce total cost of ownership. |
© 2026 Professional Dental Equipment Guide. For authorized distribution only. Specifications subject to change without notice.
Need a Quote for Dental Mobile Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


