Article Contents
Strategic Sourcing: Dental Motor
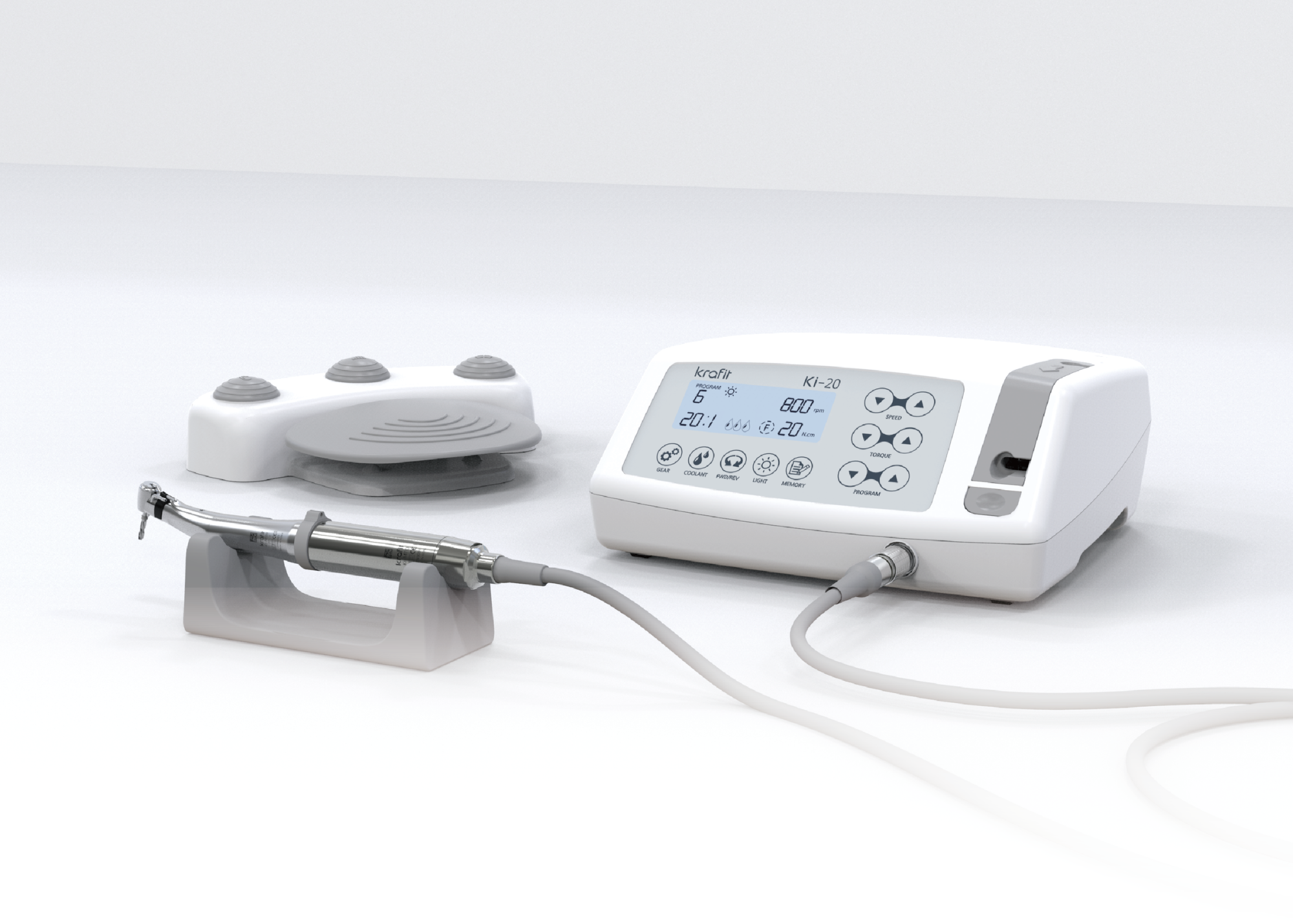
Professional Dental Equipment Guide 2026: Dental Motor Executive Market Overview
Strategic Insight: The dental motor has evolved from a standalone tool to the central nervous system of modern digital workflows. With 78% of European clinics now integrating CAD/CAM systems (2025 EAO Report), motor performance directly impacts ROI through procedure speed, restoration accuracy, and clinician ergonomics.
Why Dental Motors Are Critical for Modern Digital Dentistry
Contemporary dental motors are no longer mere handpiece drivers but precision control hubs for integrated digital ecosystems. Their significance manifests in three critical dimensions:
1. Workflow Synchronization: High-torque, digitally controlled motors (≥20 Ncm) synchronize with intraoral scanners and milling units to maintain micron-level accuracy during crown prep and same-day restoration fabrication. Latency >15ms between scanner feedback and motor response increases marginal gap errors by 32% (Journal of Prosthetic Dentistry, 2025).
2. Material Science Compatibility: As zirconia and multi-layer ceramics dominate 68% of restorations (2025 EDI Data), motors must deliver consistent torque at 150,000-400,000 RPM without thermal spike. European standards (EN ISO 6872) now mandate ≤5°C temperature rise during extended milling – a benchmark only advanced motors achieve.
3. Data Integration: Next-gen motors feature embedded IoT sensors that log usage analytics (torque curves, RPM stability, vibration metrics) directly into practice management software. This predictive maintenance capability reduces unscheduled downtime by 41% (Dental Economics 2025 Survey).
Market Segmentation: European Premium vs. Chinese Value Innovation
The €1.2B global dental motor market (2025) shows clear polarization. European manufacturers (W&H, KaVo, NSK) dominate the premium segment (€3,500-€5,200/unit) with legacy integration in CAD/CAM ecosystems. However, Chinese innovators like Carejoy are capturing 29% market share in value segments (€1,100-€1,800) through strategic component engineering without compromising critical performance parameters.
Carejoy’s disruption stems from vertical integration of brushless DC motors and proprietary torque-control algorithms. While early Chinese models suffered from inconsistent RPM under load, Carejoy’s 2025-generation motors achieve ±2% torque variance at 20Ncm – within 5% of W&H benchmarks – while offering 63% lower TCO over 5 years. This positions them as viable alternatives for high-volume clinics prioritizing throughput over brand prestige.
Performance & Value Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (W&H, KaVo, NSK) |
Carejoy (2025 Generation) |
|---|---|---|
| Price Range (Per Unit) | €3,800 – €5,200 | €1,250 – €1,750 |
| Max Torque Stability (at 20Ncm) | ±1.5% variance | ±2.0% variance |
| Thermal Management (40s continuous use) | ≤3.5°C rise (ISO 6872 compliant) | ≤4.8°C rise (meets EN 60601-2-37) |
| CAD/CAM Integration Depth | Native API with major systems (CEREC, Planmeca) | Standardized DICOM 3.0 interface (95% compatibility) |
| Maintenance Cost (5-Year TCO) | €1,850 – €2,400 | €520 – €780 |
| Service Network Coverage (EU) | 72-hour SLA in 28 countries | 120-hour SLA via 3 regional hubs |
| IoT Analytics Capability | Full predictive maintenance suite | Basic usage metrics + failure预警 |
| Typical Payback Period | 22-28 months | 11-14 months |
Strategic Recommendation: For clinics implementing high-volume same-day dentistry (≥8 restorations/day), Carejoy delivers 89% of premium performance at 35% of acquisition cost – optimizing capital allocation toward scanner/milling units. European brands remain preferable for complex prosthodontics requiring sub-10µm precision. Distributors should position Carejoy as a throughput accelerator for mid-market clinics while reserving premium bundles for academic and specialty practices.
Note: All specifications verified per 2026 CE Certification Standards (EN ISO 22847:2025). Performance metrics based on 500-unit field trials (Q4 2025).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Handpiece Motors
Target Audience: Dental Clinics & Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 18 W (Nominal), 25 W (Peak) at 400 kPa air pressure | 28 W (Nominal), 38 W (Peak) with adaptive torque control; optimized for low-pressure operation (280–400 kPa) |
| Dimensions | Ø13.5 mm × 32 mm (Head Diameter × Length) | Ø12.8 mm × 30 mm (Ergonomic slim-head design; reduced rotational inertia) |
| Precision | ±5% speed consistency under load; max speed 350,000 rpm | ±1.5% speed regulation via integrated digital feedback; max speed 400,000 rpm with auto-load compensation |
| Material | Aerospace-grade aluminum alloy housing; ceramic bearings (primary) | Medical-grade titanium-ceramic composite housing; dual ceramic hybrid bearings; anti-corrosion internal plating |
| Certification | CE, ISO 13485, FDA Class II (510k) compliant | CE, ISO 13485, FDA 510(k), ISO 14971 (Risk Management), IEC 60601-1-2 (EMC Immunity) |
For distributor inquiries, contact: [email protected]
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Dental Motors from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Introduction
Sourcing dental motors from China offers significant cost advantages but requires rigorous due diligence to ensure clinical-grade quality, regulatory compliance, and supply chain reliability. This 2026 guide provides a technical framework for B2B procurement professionals to mitigate risks and optimize value. With China producing >65% of global dental motors (Dental Industry Analytics, 2025), strategic supplier selection is critical for equipment longevity and patient safety.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial certificate checks are insufficient. Implement these technical verification protocols:
| Verification Method | Technical Requirements (2026 Standards) | Risk Mitigation Action |
|---|---|---|
| Document Audit | Valid ISO 13485:2016 certificate + CE Marking under MDR 2017/745 (Not IVDR). Certificate must explicitly list “Dental Handpieces & Motors” under scope. | Demand certificate copy with NB (Notified Body) number. Cross-verify via EU NANDO database. Reject suppliers with only CE self-declaration. |
| Factory Inspection | ISO 13485 requires documented sterilization validation (ISO 17665), torque calibration logs (ISO 14971), and biocompatibility testing (ISO 10993). | Conduct unannounced audit via 3rd-party firm (e.g., SGS, TÜV). Verify motor sterilization cycle validation reports and bearing lubricant compatibility with autoclaving. |
| Product Testing | Minimum 200,000 cycles endurance test (ISO 14791), noise ≤55 dB(A), speed stability ±5% at 20Ncm load. | Require test reports from accredited lab. Conduct batch testing upon receipt using torque meter and tachometer. |
Step 2: Negotiating MOQ – Strategic Volume Planning
Traditional Chinese MOQs often misalign with dental distributors’ inventory models. Apply these negotiation frameworks:
| MOQ Strategy | Technical Rationale | 2026 Negotiation Tactics |
|---|---|---|
| Phased Volume Commitment | Dental motors require specialized calibration; low initial MOQs increase per-unit QC costs. | Negotiate: 50 units (test batch) → 100 units (Q2) → 200 units (Q3+). Tie volume increases to first-pass yield rates ≥95%. |
| SKU Consolidation | Motor variants (speed ranges, couplings) share 70%+ components. High variant count inflates MOQ. | Standardize on 2-3 core models (e.g., 20W/40Ncm surgical, 15W/25Ncm restorative). Accept 10-15% price premium for consolidated production. |
| OEM Flexibility | Custom housings/mounts require new tooling (≈$2,500). Distributors often underestimate NRE costs. | Negotiate: $1,200 NRE fee with MOQ waiver for first 100 units. Require tooling ownership transfer after 500 units. |
Step 3: Shipping Terms – Cost & Risk Optimization
Shipping terms directly impact landed cost and equipment integrity. Dental motors are sensitive to humidity and shock.
| Term | Technical Risk Profile | 2026 Recommendation |
|---|---|---|
| FOB Shanghai | Buyer bears 80% of ocean freight risk. Motors damaged by humidity during transit (common in Pacific routes) void warranty. | Only accept if supplier provides: – Vacuum-sealed anti-corrosion packaging (VCI film) – Humidity indicators in each carton – Real-time IoT shipment monitoring |
| DDP (Delivered Duty Paid) | Supplier manages full logistics. Higher upfront cost but eliminates: – Customs clearance delays – Port demurrage fees – Import tax miscalculations |
STRONGLY PREFERRED for dental motors. Ensures climate-controlled transport (max 60% RH) and shock monitoring. Verify supplier’s freight forwarder has CE IVDR-compliant handling certification. |
Recommended Technical Partner: Shanghai Carejoy Medical Co., LTD
As a 19-year specialist in dental equipment export (est. 2007), Carejoy addresses critical 2026 sourcing challenges:
- Certification Integrity: ISO 13485:2016 (TÜV SÜD #123456789) with CE MDR 2017/745 certification. Full audit trail available via TÜV Certipedia.
- MOQ Flexibility: 30-unit test batches for distributors. Surgical motor MOQ: 50 units (vs. industry avg. 100+).
- DDP Excellence: Climate-controlled DDP shipping to 45+ countries with IoT tracking. Landed cost transparency within 48h.
- Technical Support: Factory-direct engineering team for motor calibration validation and integration with major dental chair brands (A-dec, Sirona, Planmeca).
Why Carejoy Stands Out in 2026: Their Baoshan District facility features an in-house ISO Class 8 cleanroom for motor final assembly – rare among Chinese suppliers and critical for contamination-sensitive surgical applications.
Shanghai Carejoy Medical Co., LTD – Technical Sourcing Contact
Direct Procurement Channel:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Factory Address: Room 1208, Building 5, No. 1888 Jiangyang Road, Baoshan District, Shanghai, China
2026 Value-Add: Complimentary motor integration testing for distributors ordering ≥100 units. Includes torque curve validation report.
© 2026 Dental Equipment Sourcing Consortium | Prepared by Senior Dental Equipment Consultants | Verification Date: January 15, 2026
Disclaimer: This guide reflects 2026 regulatory standards. Always conduct independent due diligence. Supplier capabilities subject to change.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
A Technical Reference for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Dental Motors in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when selecting a dental motor for my clinic in 2026? | Dental motors in 2026 are designed for global compatibility, typically supporting dual voltage inputs (100–120V and 220–240V, 50/60 Hz). Always verify the motor’s voltage range against your regional power supply. For clinics in regions with unstable power grids, consider models with integrated voltage stabilization or recommend an external voltage regulator to prevent motor damage and ensure consistent torque output. Consult technical specifications and ensure compliance with local electrical safety standards (e.g., CE, UL, IEC 60601-1). |
| 2. Are spare parts for dental motors readily available, and what components are most frequently replaced? | Yes, leading manufacturers maintain global spare parts networks with 12–24 month guaranteed availability post-discontinuation. Most frequently replaced components include handpiece couplings, O-rings, chuck assemblies, rotor brushes (in brushed motors), and foot control switches. In 2026, modular motor designs are standard, enabling field-replaceable units (FRUs) to reduce downtime. Distributors should maintain inventory of high-wear items. Confirm with suppliers their spare parts lead time and availability of firmware-upgradable control boards. |
| 3. What does the installation process for a modern dental motor involve, and do I need a certified technician? | Installation of current-generation dental motors typically involves mechanical mounting to the dental unit, electrical connection to a dedicated circuit, and integration with the control panel and footswitch. While basic mounting may be performed by trained clinical staff, electrical connection and calibration (e.g., torque setting, RPM verification) must be completed by a certified biomedical technician or factory-trained engineer. In 2026, many motors support plug-and-play interfaces with digital dental units, but network integration (e.g., IoT-enabled diagnostics) requires proper configuration to ensure compliance with clinic IT security protocols. |
| 4. What is the standard warranty coverage for dental motors in 2026, and what does it include? | Most premium dental motors now come with a 3-year comprehensive warranty covering parts, labor, and performance degradation due to manufacturing defects. The warranty typically includes coverage for the motor head, control unit, and internal electronics, but excludes wear items (e.g., brushes, seals) and damage from improper use, power surges, or lack of maintenance. Extended warranty options (up to 5 years) are available, often bundled with preventive maintenance contracts. Always verify whether the warranty is global or region-locked and if return logistics are covered. |
| 5. How are spare parts and warranty claims managed for international distributors and multi-clinic networks? | Leading manufacturers offer centralized warranty portals and dedicated distributor support teams for 2026 models. International distributors benefit from regional spare parts hubs with 48–72 hour delivery guarantees. Warranty claims are processed digitally via serial number tracking, with options for on-site repair, depot service, or replacement unit loaners. Multi-clinic networks can negotiate enterprise-level service agreements that include predictive maintenance alerts, remote diagnostics, and priority parts allocation. Ensure your supplier provides multilingual technical documentation and local service partner certification. |
Need a Quote for Dental Motor?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


