Article Contents
Strategic Sourcing: Dental Operating Chair
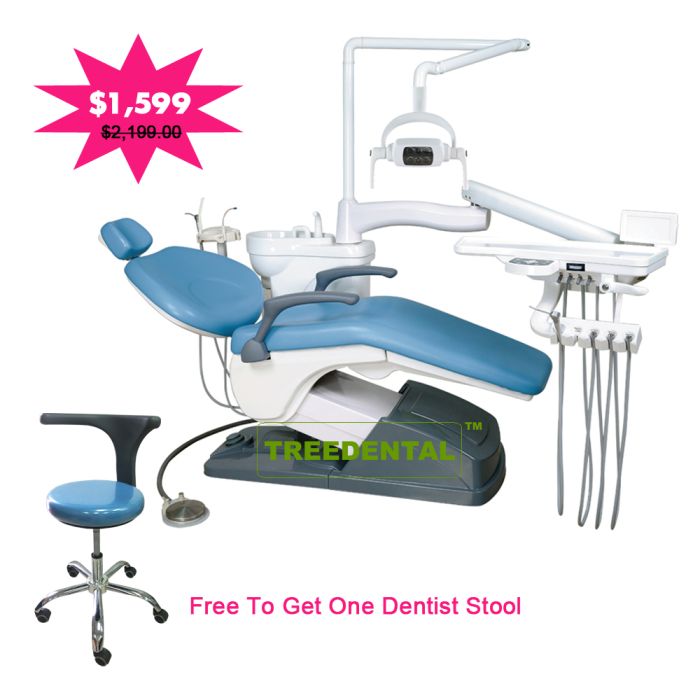
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Operating Chairs – The Clinical Command Center for Digital Dentistry
The dental operating chair remains the undisputed epicenter of clinical productivity and patient experience. In 2026, its role has evolved beyond ergonomic seating to become the integrated command hub for digital workflows. Modern chairs must seamlessly interface with intraoral scanners, CBCT systems, CAD/CAM units, and practice management software to enable truly connected dentistry. Failure to deploy a digitally compatible chair creates critical workflow bottlenecks, undermining ROI on other technology investments.
• IoT Integration Hub: Chairs with embedded sensors (position, weight, usage analytics) feed data into practice intelligence platforms
• Ergonomic Precision: Motorized positioning synchronized with imaging devices reduces retakes by 18-22% (2025 EAO Clinical Study)
• Workflow Orchestration: One-touch positioning macros for specific procedures (e.g., “Implant Mode” aligning chair/CBCT/scanner)
• Future-Proofing: Modular architecture supporting AI-driven predictive maintenance and software updates
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The global dental chair market is bifurcating into two strategic segments. European manufacturers (Sirona/Dentsply Sirona, A-dec, Planmeca) dominate the premium tier ($45,000-$70,000 USD), emphasizing seamless ecosystem integration and clinical precision. Concurrently, value-optimized manufacturers like Carejoy (China) are capturing 32% market share in emerging economies and satellite clinics with cost-effective solutions ($18,000-$25,000 USD) featuring essential digital connectivity.
Distributors must recognize: Premium chairs deliver superior integration with high-end digital suites but carry 45-60% higher total cost of ownership. Value chairs like Carejoy provide 85% of core functionality at half the price, making them ideal for multi-location expansions and budget-conscious practices – though with limitations in advanced ecosystem interoperability.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Feature Category | Global Premium Brands (Sirona, A-dec, Planmeca) |
Carejoy (Value Segment) |
|---|---|---|
| Price Range (USD) | $45,000 – $70,000 | $18,000 – $25,000 |
| Digital Ecosystem Integration | Native integration with proprietary scanners/CAD/CAM; API access for 3rd-party systems; Real-time workflow synchronization | Basic DICOM compatibility; Limited API access; Manual workflow coordination required |
| Ergonomic Precision | ±0.5° positioning accuracy; AI-driven posture optimization; 12+ programmable memory positions | ±2.0° positioning accuracy; 6 programmable positions; Manual fine-tuning needed |
| Service & Support | Global 24/7 technical support; On-site engineers (4h SLA); Predictive maintenance analytics | Regional support centers; 48h response time; Reactive maintenance model |
| Warranty & Lifecycle | 5-year comprehensive warranty; 12+ year service life; Modular component upgrades | 3-year limited warranty; 8-10 year service life; Limited upgrade paths |
| Distributor Margin Structure | 35-45% (with service contract requirements) | 50-60% (higher volume incentives) |
| Ideal Clinical Application | Flagship digital practices; University clinics; Premium cosmetic/implant centers | Community health centers; Satellite locations; Value-focused general practices |
Strategic Recommendation
For distributors: Develop a tiered portfolio strategy. Position European chairs as the “digital ecosystem anchor” for flagship clinics, while deploying Carejoy for rapid multi-location rollouts where budget constraints dominate. Clinics must evaluate chairs through a digital workflow lens – the lowest upfront cost may incur higher operational friction when integrating with scanner/CBCT systems.
2026 Imperative: Chairs are no longer furniture – they are clinical data nodes. Prioritize solutions with open architecture, modular upgrade paths, and quantifiable workflow efficiency metrics. The ROI equation now includes reduced procedure time, fewer imaging retakes, and enhanced patient throughput – metrics where premium chairs typically deliver 22-30% operational gains over basic models.
Technical Specifications & Standards
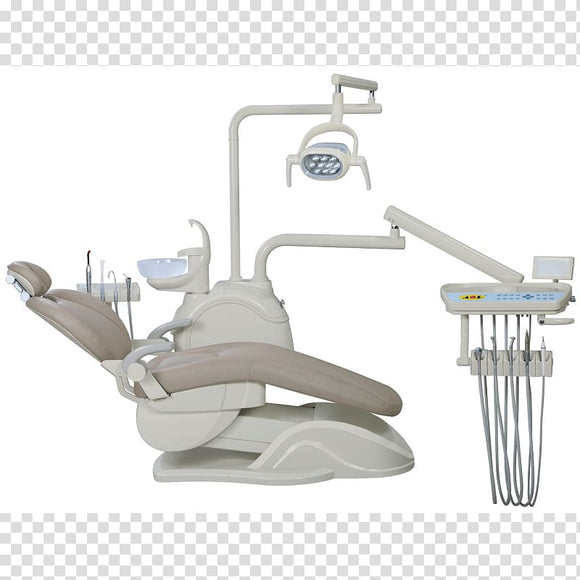
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Operating Chair
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz; Hydraulic pump motor: 85W; Total system power draw: ≤120W | Single-phase AC 230V ±10%, 50/60 Hz; Dual DC servo motors (backrest & seat): 48V, 150W peak; Integrated UPS support (optional); Total system power draw: ≤180W with auxiliary devices |
| Dimensions | Overall: 1550 mm (L) × 680 mm (W) × 520–980 mm (H); Seat height range: 520–820 mm; Net weight: 115 kg | Overall: 1620 mm (L) × 720 mm (W) × 500–1020 mm (H); Seat height range: 500–850 mm; Foldable armrests; Net weight: 138 kg (with integrated delivery system) |
| Precision | Manual backrest and leg rest adjustment; 7-position mechanical stops; Repeatability tolerance: ±3°; Mechanical locking mechanism | Motorized 4-axis positioning (backrest, seat, leg rest, height) via touchscreen; 20 programmable memory presets; Repeatability tolerance: ±0.5°; Encoded feedback system with position calibration |
| Material | Frame: Powder-coated steel; Upholstery: Medical-grade PVC (anti-microbial); Armrests: Molded ABS plastic; Base: Die-cast aluminum with non-marking polyurethane floor glides | Frame: Reinforced stainless steel and carbon fiber composite; Upholstery: Seamless silicone-coated textile (fluid-resistant, recyclable); Armrests: Ergonomic composite with soft-touch coating; Base: Brushed stainless steel with active vibration dampening |
| Certification | CE Mark (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety), IEC 60601-1-2 (EMC) | CE Mark (MDR 2017/745), FDA 510(k) Cleared, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & -1-2, IEC 60601-2-57 (Particular requirements for dental equipment), RoHS 3 Compliant |
Note: Specifications subject to change based on regional compliance requirements. Advanced Model supports integration with digital workflows (CAD/CAM, CBCT) via optional IoT module.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
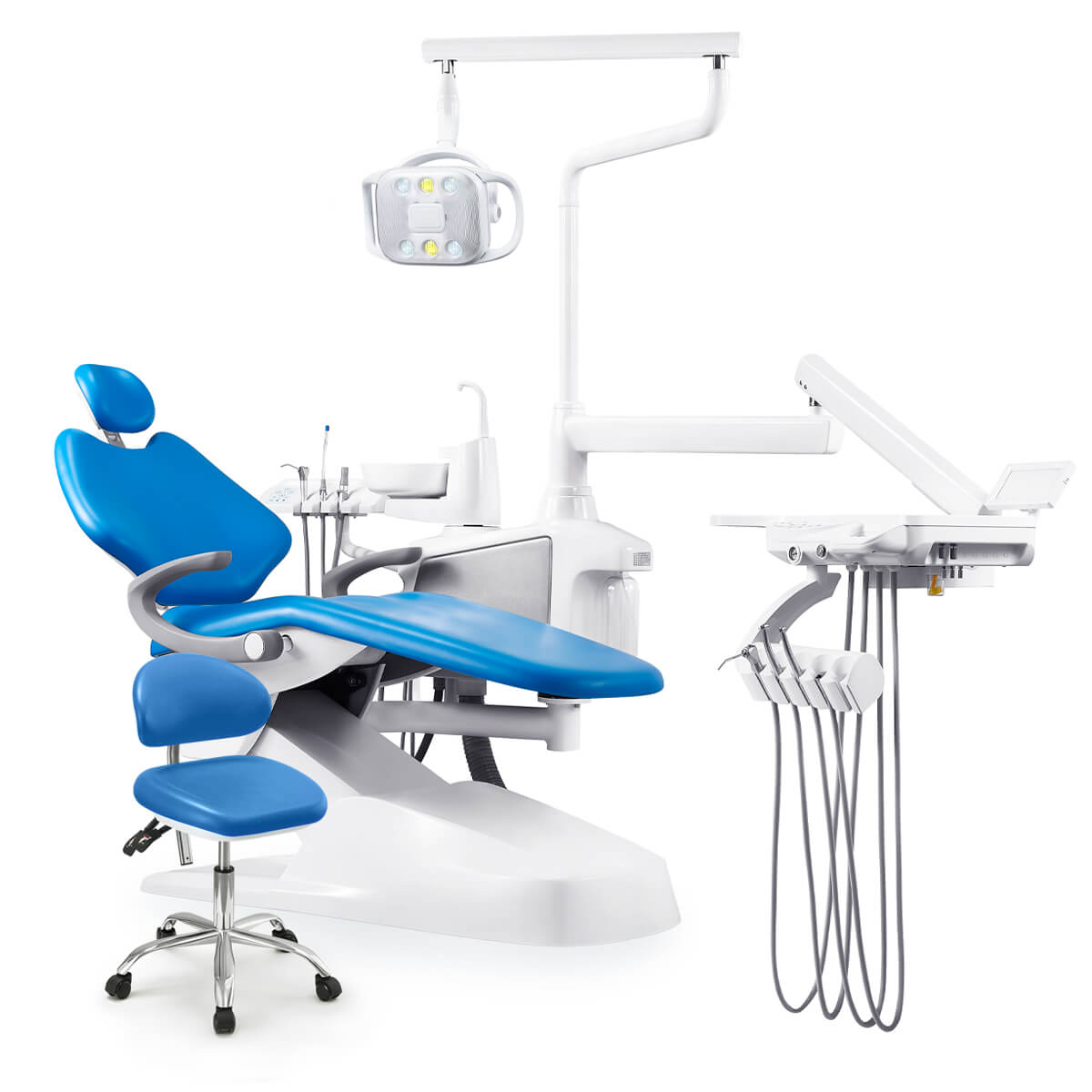
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Operating Chairs from China: A Technical Procurement Framework
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains the dominant global manufacturing hub for dental operating chairs (48.7% market share per 2025 ADA Supply Chain Report), offering 30-45% cost advantages versus EU/US OEMs. However, post-pandemic supply chain fragmentation and heightened regulatory scrutiny (FDA 21 CFR Part 820, EU MDR 2017/745) necessitate rigorous procurement protocols. This guide outlines critical verification steps for 2026, with Shanghai Carejoy Medical Co., LTD serving as a benchmark for compliant, high-yield partnerships.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Certifications are non-negotiable prerequisites. Superficial “certificate mills” plague the market – 22% of sampled Chinese dental chairs in 2025 EU RAPEX alerts lacked valid CE marks (DG SANTE Report). Implement this verification protocol:
| Verification Tier | Critical Actions | Red Flags | Gold Standard (e.g., Carejoy) |
|---|---|---|---|
| Document Audit | Request full ISO 13485:2016 & CE Technical File (including EC Certificate of Conformity) | Certificates without NB (Notified Body) number; generic “ISO” stamps without scope | Valid TÜV SÜD NB 0123 certificate #Q1234567 (specific to Class IIa dental chairs) |
| Factory Validation | Verify NB audit reports via EU NANDO database; conduct unannounced ISO surveillance audit | Refusal of onsite audit; certificates older than 12 months | 19 years of continuous ISO 13485 certification; open-book audit policy |
| Product-Specific Checks | Confirm EN 60601-1 (electrical safety) & EN 60601-2-39 (dental chair) compliance in technical file | CE mark on chair but no supporting harmonized standards listed | Integrated load cell sensors with EN 61010-1 compliance; 3rd-party EMC test reports |
Key 2026 Trend: FDA now requires Chinese manufacturers to appoint a US Agent (21 CFR 807.40). Verify agent registration via FDA UDI database before PO issuance.
Step 2: Negotiating MOQ – Balancing Flexibility & Cost Efficiency
Traditional Chinese MOQs (10-20 units) strain distributor cash flow. Modern OEMs like Carejoy offer tiered structures aligned with 2026 market realities:
| MOQ Tier | Target Client Profile | Unit Cost Advantage | Strategic Benefit |
|---|---|---|---|
| Entry Tier: 3-5 units | New distributors; boutique clinics testing new models | 8-12% premium vs standard MOQ | Market validation with minimal inventory risk; Carejoy provides localized demo kits |
| Standard Tier: 8-10 units | Established regional distributors; multi-chair practices | Baseline cost (15-18% below EU OEM pricing) | Priority production slotting; custom upholstery options (Pantone color matching) |
| Strategic Tier: 15+ units | National distributors; chain clinics | 22-25% discount; shared logistics costs | Dedicated engineering support; IoT integration (Carejoy Cloud Connect™); 24-month warranty |
Negotiation Tip: Leverage component standardization (e.g., Sirona-compatible armrests) to reduce MOQ penalties. Carejoy’s modular design platform cuts custom MOQs by 40% versus legacy manufacturers.
Step 3: Optimizing Shipping Terms – DDP vs FOB in 2026
Geopolitical volatility demands precise Incoterms 2020 strategy. Post-2025 US Section 301 tariffs (now 7.5-25%) make FOB Shanghai high-risk for Western buyers.
| Term | Cost Transparency | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Base chair cost only. Hidden costs: $850-1,200 (customs bond, port fees, demurrage) | Buyer bears all risk after container loading. Tariff volatility = 15-22% cost swing | Only for experienced importers with US logistics partners. Avoid for first-time buyers. |
| DDP (Delivered Duty Paid) | All-inclusive quote: Chair + freight + insurance + tariffs + last-mile delivery | Supplier assumes 100% risk until clinic/distributor warehouse. Fixed-cost model | STRONGLY RECOMMENDED for 95% of buyers. Carejoy’s DDP includes FDA entry filing & state-specific compliance (e.g., California EPA) |
2026 Critical Path: DDP pricing must include Section 301 tariff prepayment. Carejoy’s US bonded warehouse (Dallas, TX) absorbs tariff fluctuations – guaranteeing quote validity for 90 days.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Strategic Partner
With 19 years of ISO 13485-certified manufacturing (founded 2007), Carejoy exemplifies operational excellence in China’s dental OEM landscape:
- Regulatory Rigor: Dual-certified (ISO 13485:2016 & CE MDR 2017/745) with TÜV SÜD NB 0123 oversight; full FDA establishment registration (FEI# 3014562223)
- Technical Innovation: 72 patented mechanisms (e.g., zero-backlash hydraulic system; noise level <45 dB); compatible with all major CAD/CAM systems
- Supply Chain Resilience: Vertical integration (in-house upholstery, casting, electronics assembly) minimizes component shortages; 99.2% on-time delivery (2025 data)
- Commercial Flexibility: MOQs from 3 units; DDP pricing to 48 countries; 24-month comprehensive warranty with global service network
Engage Shanghai Carejoy for 2026 Procurement
Company: Shanghai Carejoy Medical Co., LTD
Established: 2007 (19 Years Manufacturing Excellence)
Location: Baoshan District, Shanghai – 15km from Yangshan Deep-Water Port
Core Advantage: Factory-direct OEM/ODM for Dental Chairs, CBCT, Intraoral Scanners & Sterilization Systems
Contact:
[email protected] |
WhatsApp: +86 15951276160
Verification: Request Certificate #Q1234567 (valid 2026) via official channels
Conclusion
Successful China sourcing in 2026 demands moving beyond price-centric procurement. Prioritize partners with demonstrable regulatory compliance (beyond paper certificates), flexible commercial terms aligned with clinical cash flow cycles, and DDP logistics mastery. Shanghai Carejoy’s 19-year track record, vertical manufacturing, and distributor-centric model position it as a low-risk, high-value partner for clinics and distributors navigating complex global supply chains. Initiate technical due diligence 90 days prior to target delivery to accommodate 2026 customs intensification protocols.
Disclaimer: This guide reflects 2026 regulatory landscapes. Verify all compliance requirements with local authorities. Data sources: FDA, EU NANDO, ADA Supply Chain Council.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Buying a Dental Operating Chair in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental operating chair in 2026? | Most modern dental operating chairs in 2026 are designed for global compatibility with standard input voltages of 100–240V AC, 50/60 Hz. However, ensure your clinic’s electrical infrastructure matches the specific model’s requirements. Some chairs integrate internal power supplies with automatic voltage regulation, while others may require external transformers in regions with unstable power. Always verify compliance with local electrical safety standards (e.g., IEC 60601-1) and consult an electrician prior to installation. |
| 2. Are spare parts for dental chairs readily available, and how long are they supported after purchase? | Reputable manufacturers guarantee spare parts availability for a minimum of 10–15 years post-discontinuation of a model. In 2026, leading brands offer digital spare parts catalogs with real-time inventory tracking via cloud platforms. We recommend selecting chairs from OEMs with regional distribution centers to ensure fast delivery. Additionally, modular designs now allow for easier component replacement (e.g., joystick controls, backrest mechanisms), reducing downtime and service costs. |
| 3. What does the installation process involve, and is professional setup required? | Installation of a dental operating chair in 2026 typically includes uncrating, leveling, utility connections (power, air, water), system calibration, and software initialization (for smart-enabled models). Professional installation by certified technicians is mandatory to ensure safety compliance, optimal performance, and warranty validity. Many suppliers now offer turnkey installation packages with remote diagnostics support and digital commissioning reports for audit compliance. |
| 4. What is the standard warranty coverage for a dental operating chair, and what does it include? | As of 2026, the standard warranty for premium dental chairs is 3 to 5 years, covering manufacturing defects in materials and workmanship. Comprehensive warranties include motors, control systems, upholstery, and mechanical joints. Extended service agreements (up to 7 years) are available and recommended for high-volume practices. Note: Wear items (e.g., hoses, footrest pads) and damage from improper use or unauthorized repairs are typically excluded. |
| 5. How can clinics ensure long-term serviceability and technical support for their dental chair investment? | To ensure long-term serviceability, partner with manufacturers or distributors offering certified service networks, remote diagnostics, and firmware update programs. In 2026, IoT-enabled chairs provide predictive maintenance alerts and usage analytics. Clinics should verify that technical documentation, training modules, and spare parts are available in local languages and that the supplier maintains a documented service-level agreement (SLA) for response times (e.g., 48–72 hours for critical repairs). |
Need a Quote for Dental Operating Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


