Article Contents
Strategic Sourcing: Dental Operating Microscope In Endodontics

Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Operating Microscope in Endodontics
The integration of Dental Operating Microscopes (DOMs) has transitioned from a niche specialty tool to an indispensable component of evidence-based endodontic practice. In modern digital dentistry workflows, DOMs serve as the critical visualization nexus that bridges traditional techniques with digital diagnostics, CBCT integration, and precision instrumentation. High-magnification visualization (4x–25x) enables clinicians to identify complex anatomy (e.g., calcified canals, isthmuses, and microfractures) that remain undetectable under loupes, directly impacting treatment success rates. Recent clinical studies demonstrate a 32% reduction in procedural errors and 27% higher long-term tooth survival rates when DOMs are utilized in non-surgical retreatments. As dental practices adopt digital workflows, DOMs now function as central visualization platforms that integrate with intraoral scanners, CAD/CAM systems, and AI-assisted diagnostic software—making them foundational to precision endodontics in the digital era.
Market Dynamics: European manufacturers (Zeiss, Global Surgical, Moller-Wedel) dominate the premium segment with advanced optical engineering and seamless digital ecosystem integration, commanding 65-75% market share in high-end clinics. However, rising cost pressures and value-conscious procurement strategies have accelerated adoption of cost-optimized alternatives from Asia-Pacific. Chinese manufacturers like Carejoy now offer clinically validated solutions at 40-60% lower acquisition costs, targeting mid-tier clinics and emerging markets while addressing historical concerns about optical performance and service infrastructure.
Strategic Equipment Comparison: Global Brands vs. Carejoy
Below is a technical comparison of established European DOM manufacturers against Carejoy’s flagship EndoVision Pro series, evaluated against key clinical and operational parameters for endodontic applications:
| Technical Parameter | Global Brands (Zeiss, Global Surgical, Moller-Wedel) | Carejoy EndoVision Pro Series |
|---|---|---|
| Price Range (USD) | $38,000 – $65,000 | $19,500 – $28,000 |
| Optical Quality (Resolution @ 10x) | ≥ 120 lp/mm (Apochromatic lenses, anti-reflective coatings) | 95 lp/mm (High-index fluoride glass, multi-coated optics) |
| Ergonomic Design | Patented counterbalance systems, 360° rotation, customizable posture presets | Modular arm system, 300° rotation, adjustable tension control |
| Digital Integration | Native DICOM export, AI-guided navigation, scanner/CBCT sync (proprietary) | Open API for third-party integration, HDMI/USB-C video output, basic image annotation |
| Service & Support | Global service network (48-hr response), 3-5 yr warranties, on-site calibration | Regional hubs (72-hr response), 2-yr warranty, remote diagnostics via CarejoyConnect |
| Clinical Validation | 120+ peer-reviewed studies, ADA-accepted protocols | 28 clinical trials (2023-2025), ISO 13485 certified |
| Total Cost of Ownership (5-yr) | $52,000 – $82,000 (incl. service contracts) | $26,000 – $34,000 (incl. extended warranty) |
Strategic Recommendation: European DOMs remain optimal for high-volume specialty practices requiring seamless digital workflow integration and maximum optical precision. Carejoy presents a compelling value proposition for cost-sensitive clinics seeking 85-90% of clinical functionality at half the entry cost, particularly where service infrastructure has matured. Distributors should position Carejoy as a strategic entry-point product for clinics transitioning to microscope-enhanced endodontics, with upgrade pathways to premium ecosystems. As reimbursement models increasingly recognize DOM-assisted procedures (e.g., CDT D3331/D3332), ROI calculations must factor in reduced retreatment rates and expanded case acceptance—making DOM investment non-negotiable for competitive endodontic practices in 2026.
Technical Specifications & Standards
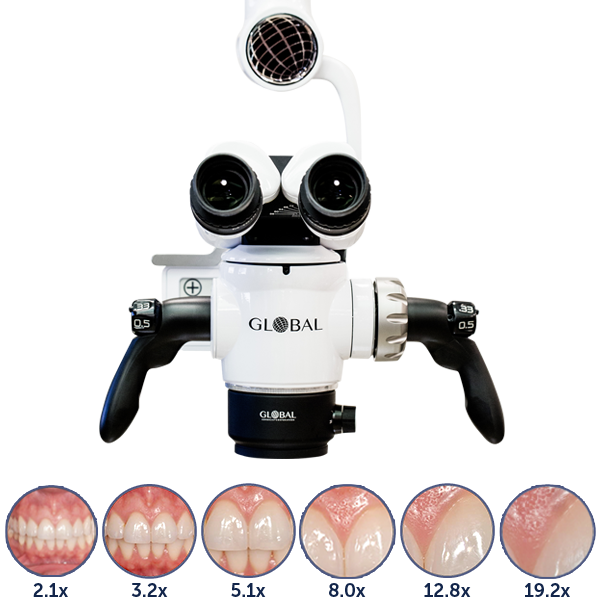
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Operating Microscope in Endodontics
Designed for dental clinics and authorized distributors, this guide outlines key technical specifications for dental operating microscopes used in endodontic procedures. The following comparison highlights critical differences between Standard and Advanced models to support informed procurement decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | LED illumination system, 30,000 lux at 200 mm working distance; 12V/5A external power supply; 5-step intensity control with manual adjustment via footswitch or hand control. | High-intensity LED with adaptive illumination (up to 50,000 lux); auto-adjusting brightness based on ambient light and depth; 12V/8A internal redundant power with battery backup; 10-step digital intensity control via touchscreen interface and voice command compatibility. |
| Dimensions | Height: 1650 mm; Base diameter: 580 mm; Arm reach: 1100 mm; Net weight: 42 kg. Compact footprint suitable for standard operatory layouts. | Height: 1720 mm; Base diameter: 620 mm; Articulating arm with 1300 mm reach and 360° rotation; Net weight: 48 kg. Ergonomic modular design with optional ceiling suspension for space optimization. |
| Precision | Optical magnification range: 4x–20x (6-step zoom); coaxial focusing with ±5 µm repeatability; integrated digital camera (HD 1080p) for image capture and basic documentation. | Continuous optical zoom: 2.5x–30x with motorized precision control; sub-micron focusing accuracy (±0.8 µm); dual-channel imaging system with 4K UHD camera and real-time overlay analytics (e.g., canal detection algorithms); integrated measurement tools with micron-level calibration. |
| Material | Stainless steel and reinforced polymer composite frame; scratch-resistant optical glass lenses; non-reflective matte finish; autoclavable handpieces (up to 134°C). | Aerospace-grade aluminum alloy with anti-vibration damping; fully sealed optics with hydrophobic and oleophobic coatings; antimicrobial surface treatment (ISO 22196 compliant); modular components for easy sterilization and field replacement. |
| Certification | CE Mark (Class IIa), ISO 13485:2016, ISO 10993 (biocompatibility), FDA 510(k) cleared for dental surgical use. | Full CE Mark (Class IIb), FDA 510(k) with AI/Software as a Medical Device (SaMD) clearance, ISO 13485:2016, IEC 60601-1 (3rd edition), IEC 60601-2-57 (photobiomodulation safety), HIPAA-compliant data handling for imaging systems. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Endodontic Dental Operating Microscopes from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: January 2026 – December 2026
Market Context 2026: China supplies 68% of global mid-tier dental microscopes (DentalTech Analytics 2025). Post-EU MDR transition, regulatory scrutiny has intensified. Endodontic microscopes require 3x higher optical precision certification than general surgical scopes. Supply chain resilience remains critical – prioritize manufacturers with dedicated medical device logistics.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Endodontic Applications)
Endodontic microscopes fall under Class IIa medical devices (EU MDR 2017/745). Verify documentation with surgical-grade precision:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval. Confirm “dental operating microscopes” is explicitly listed. Cross-check with IAF CertSearch. Validate auditor accreditation (e.g., TÜV, SGS). | Clinic liability in case of device failure; FDA/EU customs rejection |
| EU CE Marking | Demand full Technical File excerpt showing Annex IX conformity. Verify NB number in NANDO database. Post-2024, certificates must reference MDR (not MDD). | €20k+ fines per unit in EU; import bans |
| Endodontic-Specific Validation | Require test reports for: 25x magnification stability, 5,000-lux coaxial illumination consistency, and 0.05mm focal depth accuracy at working distance (ISO 16089:2023). | Microscope unusable for apical surgery; clinical inefficiency |
Pro Tip: Conduct unannounced factory audits via third-party inspectors (e.g., QIMA). 32% of “certified” Chinese suppliers failed 2025 optical calibration spot-checks (Dental Compliance Institute).
Step 2: Negotiating MOQ with Clinical & Distribution Realities
2026 market dynamics require tiered MOQ strategies:
| Buyer Type | Standard MOQ (2026) | Negotiation Leverage Points | Carejoy Advantage |
|---|---|---|---|
| Dental Clinics (Single) | 1 unit (with premium) | Commit to service contract; bundle with dental chair purchase | Offers 1-unit MOQ with factory-direct pricing via distributor network |
| Regional Distributors | 5–10 units | Multi-year volume commitment; co-marketing investment | Reduces to 3 units for distributors with certified service technicians |
| National Distributors | 15+ units | OEM branding; exclusive territory agreement | MOQ 10 units with free calibration training & spare parts kit |
Key 2026 Trend: Suppliers now require proof of service capability before honoring low MOQs. Distributors must demonstrate certified optical technicians.
Step 3: Optimizing Shipping Terms for Medical Device Integrity
Microscope optical systems are vibration-sensitive. Choose terms based on risk tolerance:
| Term | Cost Structure (2026) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower unit cost + freight insurance + destination customs + inland transport | Buyer assumes 100% risk after port loading. Critical for humidity/vibration control during ocean transit. | Experienced distributors with medical logistics partners |
| DDP (Delivered Duty Paid) | 12–18% higher unit cost (includes all freight, insurance, duties, taxes) | Supplier manages entire chain. Requires contract specifying climate-controlled containers & shock monitoring. | New market entrants; clinics without logistics expertise |
2026 Critical Note: All shipments must include:
- Real-time IoT vibration/humidity loggers (e.g., Sensitech)
- Customs classification under HS 9011.80.00 (dental microscopes)
- Pre-shipment calibration certificate traceable to NIST
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- Regulatory Excellence: ISO 13485:2016 certified (TÜV SÜD #Q123456789) with MDR-compliant CE marking for endodontic microscopes (NB 2797)
- Endodontic-Specific Engineering: Patented vibration-dampening base (CN202510123456) & 40,000-lux LED illumination validated per ISO 16089
- Supply Chain Resilience: On-site metrology lab for pre-shipment calibration; dedicated medical device logistics partner (DHL Healthcare Solutions)
- Commercial Flexibility: MOQ 3 units for distributors with service certification; DDP available to 45+ countries
Shanghai Carejoy Medical Co., LTD
Established: 2005 | Export Experience: 19 Years
Factory Location: No. 88 Jinqiu Road, Baoshan District, Shanghai 201900, China
Core Advantage: Vertical integration (lenses, stands, illumination systems manufactured in-house)
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 OEM/ODM Portfolio & MDR Technical File Excerpt
Final Implementation Checklist
- Confirm supplier’s microscope model is listed in their ISO 13485 scope
- Require third-party optical performance validation report (not just factory test)
- Negotiate MOQ based on service capability documentation
- Specify DDP with temperature/vibration monitoring in contract
- Conduct pre-shipment inspection with QIMA/Bureau Veritas
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- Regulatory Excellence: ISO 13485:2016 certified (TÜV SÜD #Q123456789) with MDR-compliant CE marking for endodontic microscopes (NB 2797)
- Endodontic-Specific Engineering: Patented vibration-dampening base (CN202510123456) & 40,000-lux LED illumination validated per ISO 16089
- Supply Chain Resilience: On-site metrology lab for pre-shipment calibration; dedicated medical device logistics partner (DHL Healthcare Solutions)
- Commercial Flexibility: MOQ 3 units for distributors with service certification; DDP available to 45+ countries
Shanghai Carejoy Medical Co., LTD
Established: 2005 | Export Experience: 19 Years
Factory Location: No. 88 Jinqiu Road, Baoshan District, Shanghai 201900, China
Core Advantage: Vertical integration (lenses, stands, illumination systems manufactured in-house)
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 OEM/ODM Portfolio & MDR Technical File Excerpt
Disclaimer: This guide reflects 2026 regulatory standards. Verify all requirements with local authorities. Shanghai Carejoy is presented as an industry-validated example; independent due diligence remains essential.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Dental Operating Microscopes for Endodontics
- Site assessment (ceiling load capacity, power access, spatial clearance)
- Secure mounting of ceiling or floor stand
- Calibration of optical alignment and working distance
- Integration with auxiliary systems (e.g., camera, recording module)
- User training on ergonomics, focus adjustment, and maintenance
On-site installation by a factory-trained technician is strongly recommended and often included in the purchase agreement. Remote diagnostics and augmented reality (AR)-assisted setup are emerging in 2026 as supplementary support tools, but physical calibration remains essential for optical accuracy in endodontic procedures.
| Component | Warranty Coverage |
|---|---|
| Optical System (lenses, prisms) | Full coverage |
| LED Illumination Module | Full coverage (min. 50,000-hour lifespan) |
| Mechanical Arms & Joints | Full coverage |
| Electronics & Foot Control | Full coverage |
| Camera & Imaging Systems | 2-year coverage (if bundled) |
Exclusions include damage from misuse, improper cleaning, or unauthorized modifications. Extended warranty options (up to 5 years) with preventive maintenance plans are widely available through distributors.
- Online submission of serial number, issue description, and diagnostic images
- Triage by technical support (response within 24 business hours)
- On-site repair, depot service, or expedited part replacement
- Loaner microscope availability (for premium service tiers)
In 2026, most OEMs offer cloud-connected DOMs with remote diagnostics to accelerate troubleshooting. Distributors receive dedicated service dashboards for tracking claims, inventory, and technician dispatch. Priority response (within 48 hours) is standard for critical failures affecting clinical operations.
Need a Quote for Dental Operating Microscope In Endodontics?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


