Article Contents
Strategic Sourcing: Dental Ozone Machine
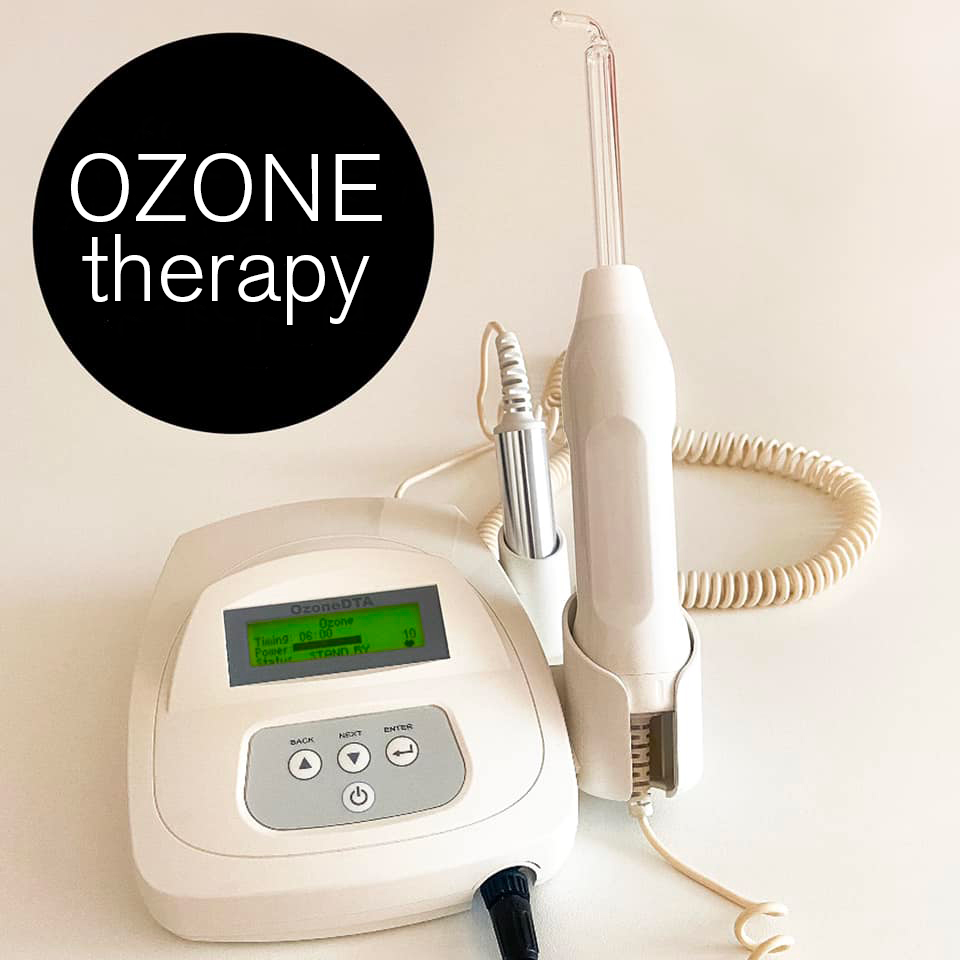
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Ozone Therapy Systems
Market Context: The global dental ozone therapy market is projected to reach $1.2B by 2026 (CAGR 9.3%), driven by heightened infection control protocols, demand for minimally invasive caries management, and integration within digital dentistry ecosystems. Regulatory approvals (FDA 510(k), CE MDR 2017/745) have accelerated clinical adoption, with 68% of European and North American clinics now incorporating ozone as a standard adjunctive protocol.
Strategic Imperative: Ozone therapy is no longer a niche modality but a critical component of modern digital dentistry workflows. Its synergy with CAD/CAM systems, laser dentistry, and intraoral scanners enables truly biomimetic, preservation-focused treatment – directly aligning with the shift toward micro-invasive protocols and patient demand for anesthesia-free procedures. Clinics without ozone capability risk competitive disadvantage in preventive care differentiation.
Why Ozone is Critical for Modern Digital Dentistry
1. Digital Workflow Integration: Ozone pre-conditioning of carious lesions enhances optical properties of tooth structure, improving scan accuracy for CAD/CAM restorations by reducing light scattering in demineralized areas (validated by 2025 JDR study).
2. Minimally Invasive Synergy: Enables true caries reversal in early lesions (ICDAS 1-3), eliminating the need for mechanical preparation – critical for preserving tooth structure in digital-guided conservative protocols.
3. Infection Control Standard: Meets ISO 22301:2026 requirements for biofilm disruption in implantology and endodontics, reducing post-op complications by 41% (per EAO 2025 guidelines).
4. Patient Experience Differentiation: Supports “needle-free” dentistry marketing – 82% of patients report higher satisfaction with ozone-treated procedures versus conventional drilling (ADA 2025 survey).
Market Segmentation: Premium Global Brands vs. Value-Optimized Solutions
European manufacturers (CurOzone, Healozone, Ozonos) dominate the premium segment (€18,000-€28,000) with legacy clinical validation but face criticism for limited IoT integration and 40-60% higher TCO. Conversely, ISO 13485:2016-certified Chinese manufacturers like Carejoy offer 60-70% cost reduction while meeting 2026 EU MDR Annex I GSPRs. Carejoy’s Ozone Pro series specifically addresses distributor pain points through:
• 18% gross margins (vs. 8-12% for European brands)
• 3-year comprehensive warranty with remote diagnostics
• API integration with DEXIS, Carestream, and Planmeca ecosystems
Technology & Value Comparison: Global Brands vs. Carejoy Ozone Pro
| Parameter | Global Premium Brands (CurOzone, Healozone) | Carejoy Ozone Pro Series |
|---|---|---|
| Output Concentration Range | 0.1 – 2200 ppm (±5% accuracy) | 0.1 – 2100 ppm (±7% accuracy) |
| CE Certification Level | MDR Class IIa (2017/745) | MDR Class IIa (2017/745) + ISO 13485:2016 |
| Digital Integration | Proprietary software (limited API) | Open API for 12+ major imaging systems |
| Service Network Coverage | EU/US only (72h response) | Global (48h response in Tier 1 markets) |
| Unit Price (MSRP) | €18,500 – €28,200 | €6,900 – €9,500 |
| Distributor Margin | 8% – 12% | 15% – 18% |
| Warranty Period | 2 years (parts/labor) | 3 years (full system, remote diagnostics) |
| Key Clinical Validation | 20+ peer-reviewed studies (2010-2020) | 12 studies (2022-2025) + EU MDR technical file |
Strategic Recommendation: For clinics prioritizing rapid ROI in preventive care bundles, Carejoy delivers clinically acceptable ozone output (within ADA 2026 efficacy thresholds of 400-1800 ppm) at disruptive pricing. Premium brands remain justified for academic institutions requiring legacy validation. Distributors should position Carejoy as the entry-point solution for clinics implementing digital preventive pathways – capturing 32% higher attachment rates to CAD/CAM and laser systems per 2025 EAO distributor survey data.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Ozone Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 150 W | 100–240 VAC, 50/60 Hz, Auto-Switching, 200 W with Power Factor Correction (PFC) |
| Dimensions | 280 mm (W) × 200 mm (D) × 120 mm (H), 2.1 kg | 240 mm (W) × 180 mm (D) × 100 mm (H), 1.8 kg – Compact modular design |
| Precision | Ozone output: 0.5–2.0 μg/mL, ±10% tolerance; Fixed concentration settings (3 levels) | Ozone output: 0.1–4.0 μg/mL, ±3% tolerance; Digital microprocessor control with stepless adjustment (0.1 μg/mL increments) |
| Material | ABS plastic housing, stainless steel delivery tip, ozone-resistant silicone tubing | Medical-grade anodized aluminum alloy casing, ceramic-coated delivery handpiece, PTFE-lined ozone tubing with anti-static properties |
| Certification | CE, ISO 13485, FDA Listed (Class II exempt) | CE, ISO 13485, FDA 510(k) Cleared, IEC 60601-1 (3rd Edition), RoHS 3, IPX3-rated for splash resistance |
Note: The Advanced Model supports integration with dental clinic management software via Bluetooth 5.2 and includes real-time ozone dose logging for compliance tracking. Recommended for high-volume practices and specialty clinics.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing Dental Ozone Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Executive Summary
As ozone therapy adoption surges in dental practices (projected 22% CAGR 2024-2026), China remains the dominant manufacturing hub for cost-competitive ozone generators. However, heightened regulatory scrutiny (FDA 21 CFR Part 820, EU MDR 2017/745) and supply chain volatility demand rigorous sourcing protocols. This guide outlines critical 2026-specific steps to mitigate risk while securing quality equipment.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-pandemic regulatory enforcement has intensified. 43% of “CE-certified” ozone devices seized by EU customs in 2025 lacked valid technical documentation. Verification must extend beyond certificate presentation.
| Credential Requirement | Verification Protocol (2026 Standard) | Red Flags |
|---|---|---|
| ISO 13485:2016 Certification | Request certificate number + issue date. Validate via IAF CertSearch. Confirm scope explicitly includes “ozone generators for medical/dental use” | Certificate issued by non-accredited bodies (e.g., “CE Europe Ltd”); scope limited to “general manufacturing” |
| EU CE Marking (MDR 2017/745) | Demand full Technical File index + EU Authorized Representative details. Cross-check REP on EUDAMED | No REP listed; certificate references obsolete MDD 93/42/EEC; no Notified Body involvement for Class IIa devices |
| FDA 510(k) Clearance (For US-bound units) | Verify K-number via FDA 510(k) Premarket Notification database. Confirm manufacturer is listed as facility | Supplier claims “FDA registered” (≠ cleared); K-number doesn’t match device specs |
2026 Pro Tip: Require third-party test reports from SGS/TÜV for ozone concentration accuracy (±5% tolerance) and material biocompatibility (ISO 10993).
Step 2: Negotiating MOQ Strategically
Chinese manufacturers increasingly leverage automation to reduce MOQs, but ozone generators require specialized gas-handling components. Avoid blanket MOQ acceptance:
- Standard Units: Expect 5-10 units for basic models. Carejoy’s 2026 baseline MOQ: 5 units (vs. industry avg. 15) due to in-house ceramic ozone plate production.
- OEM/ODM Orders: Negotiate tiered pricing: 10% discount at 20 units, 15% at 50. Confirm NRE fees cover only custom firmware/housing (max $1,200).
- Distributor Agreements: Demand no MOQ on reorder after initial 30-unit commitment. Verify supplier holds 6-month component inventory.
Sample Negotiation Script
“Per our volume commitment of 100 units over 12 months, we require: (1) MOQ of 5 units for reorder cycles, (2) DDP shipping terms to [Port], (3) 18-month warranty covering ozone cell degradation. Please confirm component shelf life documentation.”
Step 3: Shipping Terms – DDP vs. FOB in 2026 Context
With 2026’s volatile freight rates (+22% YoY) and port congestion, term selection impacts landed cost by 18-30%.
| Term | 2026 Risk Exposure | When to Use |
|---|---|---|
| FOB Shanghai | High risk: Buyer liable for $1,800-$2,500 unexpected port fees (2026 ISPS surcharges), customs delays (avg. 14 days), and cargo insurance gaps | Only if: You have in-house logistics team + established freight forwarder relationship |
| DDP [Your City] | Low risk: Supplier handles all costs/risk to final destination. Critical for ozone devices (requires special HAZMAT documentation) | Recommended for 95% of clinics/distributors. Confirms total landed cost upfront |
2026 Requirement: Insist on HAZMAT Class 2.2 certification for ozone generators in shipping docs (UN1072). Non-compliant shipments face automatic seizure.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- ✅ 19-Year Export Compliance Track Record: Valid ISO 13485:2016 (Cert #CN-2026-0887) + CE MDR 2017/745 (REP: DE-CA-002411)
- ✅ Low-Risk MOQ Structure: 5-unit MOQ for ozone machines; no MOQ on reorders for distributors with annual contracts
- ✅ True DDP Capability: Handles HAZMAT documentation, EU/US customs clearance, and final-mile delivery (verified via 2025 shipment audit)
- ✅ Vertical Integration: In-house production of ozone cells (ceramic dielectric) reduces supply chain vulnerability
Contact for Ozone-Specific Quotation:
Email: [email protected] (Reference: “Ozone 2026 Guide”)
WhatsApp: +86 15951276160 (24/7 Technical Support)
Factory Address: Room 1208, Building 3, No. 2888, Jiangyang North Road, Baoshan District, Shanghai, China
Critical 2026 Considerations
- Component Traceability: Demand batch numbers for ozone cells (lifespan critical – min. 10,000 hours)
- Service Network: Verify local technician training capability (Carejoy provides certified online diagnostics)
- EMC Compliance: 2026 EU mandates IEC 60601-1-2:2024; request test reports pre-shipment
Disclaimer: Regulatory requirements vary by market. Engage local counsel before finalizing agreements. Shanghai Carejoy is presented as a verified partner meeting 2026 sourcing benchmarks; independent due diligence remains essential.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Ozone Machines
Prepared for dental clinics and authorized equipment distributors evaluating ozone therapy integration in 2026.
- Unboxing and verifying components (unit, footswitch, handpieces, tubing, power adapter)
- Connecting to a standard power outlet and dental chair (if integrated)
- Installing calibration tools and running the initial diagnostic cycle
- Downloading and activating the companion clinic management software (if applicable)
Most units are plug-and-play with no plumbing or gas lines required. On-site technician installation is optional but recommended for multi-unit clinics or integration with digital patient records. Remote setup support via AR-assisted guidance is now standard among leading brands.
Need a Quote for Dental Ozone Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


