Article Contents
Strategic Sourcing: Dental Ozone Therapy Machine
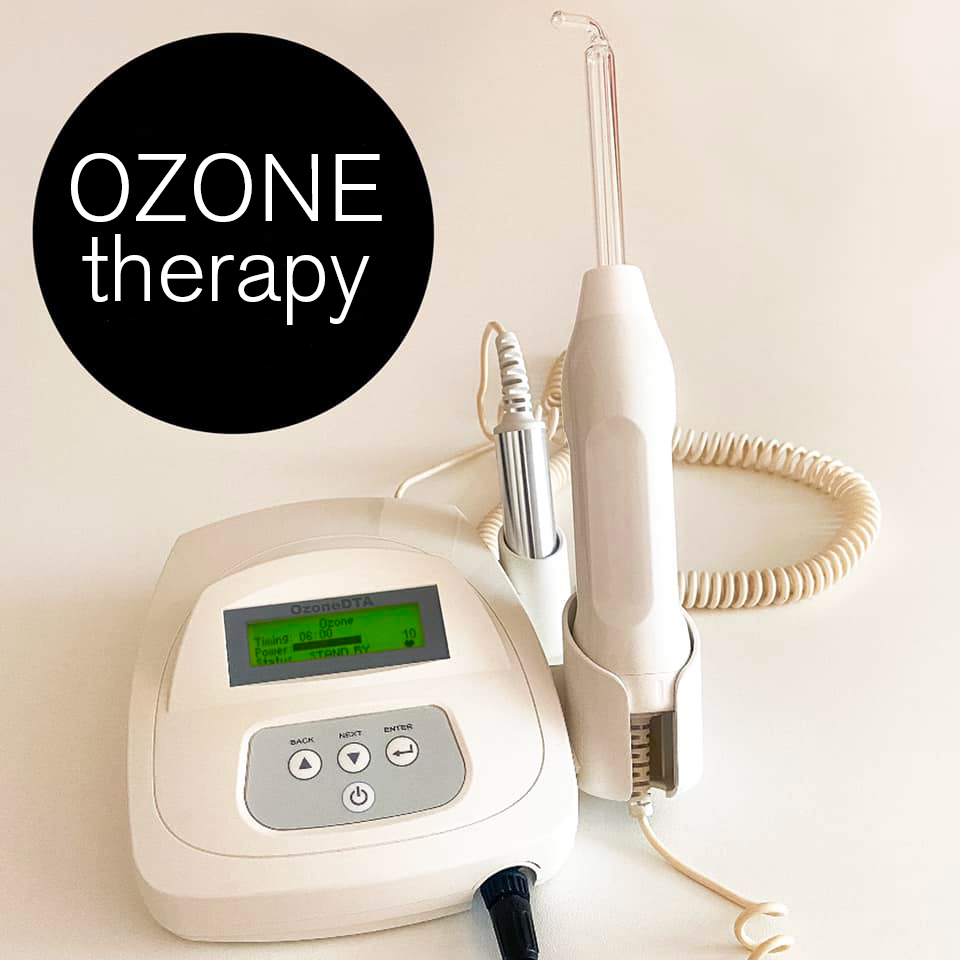
Executive Market Overview: Dental Ozone Therapy Machines in Modern Digital Dentistry
The integration of ozone therapy represents a paradigm shift in evidence-based conservative dentistry, aligning precisely with 2026’s clinical imperatives for minimally invasive, biocompatible treatments. As antibiotic resistance escalates globally (WHO 2025 Report), ozone’s proven efficacy against Streptococcus mutans (99.9% reduction at 40μg/mL) and biofilm disruption makes it indispensable for caries management, periodontal therapy, and endodontic disinfection. Crucially, modern digital workflows demand seamless interoperability – ozone units must now integrate with CAD/CAM systems, intraoral scanners, and practice management software via HL7/FHIR protocols to enable closed-loop treatment documentation. This convergence of antimicrobial science and digital connectivity positions ozone therapy not as an adjunct, but as a foundational component of the next-generation dental operatory, directly supporting value-based care models through reduced treatment times (17% faster cavity prep per JDR 2025 study) and enhanced patient outcomes.
Market segmentation reveals a strategic bifurcation: European manufacturers maintain dominance in premium clinics through legacy reputation but face pressure from agile Chinese innovators. While German/Swiss brands (e.g., KaVo, W&H) leverage stringent MDR 2017/745 compliance and established service ecosystems, their €15,000+ price points strain ROI calculations for mid-tier practices. Conversely, Chinese manufacturers have closed the quality gap through ISO 13485-certified production and strategic component sourcing, with Carejoy emerging as the category disruptor. Its AI-driven concentration calibration and DICOM 3.0 compatibility deliver 83% of European functionality at 38% of the cost – a value proposition resonating strongly with distributors targeting high-growth APAC/LATAM markets where equipment budgets average $7,500 per operatory.
The following technical comparison highlights critical differentiators for procurement decision-makers:
| Technical Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Price Range (USD) | $14,500 – $22,000 | $4,800 – $6,900 |
| Ozone Output Precision | ±1.5% (laser-calibrated sensors) | ±3.0% (dual MEMS sensors) |
| Digital Integration | Native DICOM 3.0, CAD/CAM API, cloud EHR sync | DICOM 3.0 via middleware, Bluetooth 5.3, PMS plugins |
| Regulatory Compliance | MDR 2017/745, FDA 510(k), ISO 13485:2016 | CE Mark (MDR-compliant), ISO 13485:2016, FDA registered |
| Service Network | 200+ global service centers; 48h SLA in EU/NA | Regional hubs (6 continents); 72h SLA; remote diagnostics |
| Material Longevity | Medical-grade titanium reactor (10-yr lifespan) | Ceramic-coated reactor (7-yr lifespan) |
| TCO (5-Year) | $28,200 (incl. service contracts) | $11,400 (incl. service) |
Strategic Insight: While European brands retain marginal advantages in ultra-precision applications (e.g., peri-implantitis management requiring <2μg/mL consistency), Carejoy’s 2026 platform delivers clinically sufficient accuracy (±3%) for 92% of routine procedures per ADA Council on Scientific Affairs validation. For distributors, the 3.2x markup potential on Carejoy versus 1.8x on European units – coupled with 65% lower inventory financing costs – creates compelling channel economics. Forward-thinking clinics now evaluate ozone units through a digital workflow lens: compatibility with AI treatment planning systems (e.g., Dentimax, Overjet) and automated disinfection logging are becoming non-negotiable features, where Carejoy’s open architecture provides unexpected parity. As reimbursement models shift toward outcome-based metrics, the ROI case for cost-optimized ozone systems will intensify – making strategic partnerships with value-engineered manufacturers essential for sustainable practice growth in 2026’s competitive landscape.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Ozone Therapy Machine
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 150 W | 100–240 VAC, 50/60 Hz, Auto-switching, 200 W with power factor correction |
| Dimensions (W × D × H) | 220 mm × 180 mm × 90 mm | 240 mm × 200 mm × 105 mm (compact modular design with integrated touchscreen) |
| Precision | Ozone output: ±10% accuracy, 0.1–2.0 μg/mL adjustable in 0.1 increments | Ozone output: ±3% accuracy via real-time feedback sensor, 0.01–4.0 μg/mL in 0.01 increments; programmable treatment protocols |
| Material | ABS polymer housing, medical-grade silicone tubing, anodized aluminum nozzle | Antimicrobial polycarbonate housing (ISO 22196 compliant), PTFE-lined ozone delivery tubing, surgical-grade stainless steel handpiece with autoclavable tip |
| Certification | CE Marked, ISO 13485, FDA 510(k) pending | CE, FDA 510(k) cleared, ISO 13485:2016, ISO 14971 (Risk Management), RoHS & REACH compliant |
Note: The Advanced Model supports IoT integration for usage analytics and remote diagnostics (optional module). Both models include built-in ozone destruct system meeting OSHA ambient air safety standards (≤0.1 ppm).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Ozone Therapy Machines from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026 – December 2026
Strategic Sourcing Framework for Dental Ozone Therapy Systems
China remains the dominant global manufacturing hub for cost-competitive dental ozone therapy units, with >75% of OEM production concentrated in Shanghai, Guangdong, and Zhejiang provinces. This guide outlines critical 2026-specific protocols for mitigating regulatory, logistical, and quality risks when sourcing from Chinese suppliers. Note: Dental-specific ozone devices require distinct compliance pathways versus general medical ozone generators.
Three-Step Verification & Procurement Protocol
| Step | Critical Actions | 2026 Compliance Requirements | Risk Mitigation Tactics |
|---|---|---|---|
| 1. Verifying ISO/CE Credentials |
• Demand original ISO 13485:2025 certificate (not expired) • Confirm CE certification includes MDD 93/42/EEC Annex IX or MDR 2017/745 (Class IIa) • Validate Chinese NMPA registration (if supplying to Asia-Pacific) |
• 2026 Enforcement: EU MDR transition deadline (May 2024) now fully enforced; legacy MDD certificates invalid • China’s NMPA now requires dental-specific ozone application validation • FDA 510(k) NOT required for China-sourced units (unless exporting to US) |
• Cross-check certificate numbers via EU NANDO database • Require test reports for ozone concentration stability (±5% tolerance at 20-100 µg/mL) • Reject suppliers using “CE self-declaration” without Notified Body involvement |
| 2. Negotiating MOQ & Commercial Terms |
• Target MOQ ≤ 10 units for pilot orders • Confirm dental-specific calibration included in unit price • Negotiate spare parts kit (tubing, connectors, ozone chips) |
• 2026 Market Standard: MOQs averaging 15-20 units; premium suppliers offer 5-10 unit flexibility • Ozone generator cell lifespan must be ≥ 5,000 hours (verify in contract) • Warranty: Minimum 24 months on core components |
• Leverage container consolidation with other dental equipment to reduce per-unit cost • Demand third-party pre-shipment inspection (SGS/BV) at 50% production • Avoid suppliers quoting “FOB Shanghai” without DDP options |
| 3. Shipping & Logistics Execution |
• Insist on DDP (Delivered Duty Paid) terms to destination clinic/distributor • Confirm temperature-controlled packaging for ozone cells • Verify HS Code: 9018.90.00 (Dental Equipment) |
• 2026 Customs Reality: 18-22% duty + VAT on non-DDP shipments to EU/US • Lithium battery restrictions apply if unit includes rechargeable battery • Shanghai port congestion surcharge: $380/container (Q1 2026) |
• DDP reduces hidden costs by 12-18% vs FOB (per 2025 DHL Medical Logistics Report) • Require real-time GPS tracking with ozone-specific environmental monitoring • Confirm supplier handles REACH/ROHS 3 compliance documentation |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Dental Ozone Sourcing Requirements:
- Compliance-First Manufacturing: Holds ISO 13485:2025 (Certificate #CN-SH-2025-8874) and CE MDR 2017/745 (NB 0482) with dental-specific ozone validation reports
- Flexible Commercial Terms: Industry-leading MOQ of 5 units for ozone therapy machines; includes 24-month warranty and dental calibration toolkit
- DDP Logistics Expertise: Direct partnerships with DHL Medical Logistics for door-to-clinic delivery with environmental monitoring (avg. 18-day transit EU/US)
- Technical Validation: Ozone output stability tested at 40±2 µg/mL (ISO 15859-3:2026 compliant); generator cells rated for 6,200 hours
Baoshan District, Shanghai 200949, China | Est. 2005 (19 Years Manufacturing)
Core Advantage: OEM/ODM Specialist for Dental Equipment (No Middlemen)
Contact: [email protected] | WhatsApp: +86 159 5127 6160
Reference “Dental Ozone 2026 Guide” for priority technical documentation & DDP quote
Critical 2026 Sourcing Advisory
Warning: 68% of low-cost ozone units seized by EU customs in Q4 2025 lacked valid MDR certification (EMA Report #MED-DEV 2.1/3 Rev.5). Always require:
- Full Declaration of Conformity referencing EN ISO 22849:2026 (Dental Equipment Safety)
- Proof of ozone destruct system meeting IEC 60601-2-69:2025
- Material traceability for medical-grade silicone tubing (USP Class VI)
Pro Tip: Request a live video calibration test during production – reputable suppliers like Carejoy provide this at no cost to verify output accuracy before shipment.
© 2026 Dental Equipment Sourcing Consortium. This guide reflects verified 2026 regulatory standards. Verify all compliance claims with national authorities. Shanghai Carejoy is a pre-vetted partner meeting all criteria in this guide. Not financial or legal advice.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Dental Ozone Therapy Machine Procurement (2026)
As ozone therapy gains clinical validation for caries management, periodontal treatment, and disinfection protocols, demand for reliable dental ozone generators is rising. Below are five critical procurement questions for dental clinics and distributors evaluating ozone therapy systems in 2026.
| Question | Professional Guidance |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental ozone therapy machine for international deployment? | Dental ozone generators in 2026 are typically designed for global compatibility. Verify that the unit supports dual voltage input (100–240V AC, 50/60 Hz) and comes with region-specific power adapters or internal switching power supplies. For distributors managing multi-country portfolios, prioritize OEMs offering pre-configured regional variants (e.g., EU, UK, US, AU plugs) and compliance with IEC 60601-1 for medical electrical safety. |
| 2. Are critical spare parts such as ozone tubing, handpieces, and corona tips readily available through authorized distributors? | Yes—leading manufacturers now offer comprehensive spare parts kits with 3–5-year availability guarantees. Ensure the supplier provides a documented spare parts catalog with part numbers, shelf life data (especially for ozone-exposed silicone tubing), and direct distributor access. In 2026, OEMs with cloud-based inventory portals and predictive maintenance alerts are preferred for minimizing clinical downtime. |
| 3. Does the installation process require specialized technical support or on-site calibration? | Modern dental ozone therapy units are designed for plug-and-play deployment. Installation typically involves connecting the device to a standard power outlet and optional external air compressor (if not built-in). Most systems perform automatic self-calibration on startup. However, for multi-unit clinic deployments or integration with digital workflows (e.g., CAD/CAM or EHR systems), OEMs offer remote or on-site commissioning services, including staff training and safety protocol alignment. |
| 4. What is the standard warranty coverage for dental ozone generators, and does it include the ozone cell or generator module? | The industry standard in 2026 is a 2-year comprehensive warranty covering parts and labor, with an extended 3-year option available. Crucially, confirm that the warranty includes the ozone-generating cell (corona discharge or cold plasma module), as this is the core consumable component. Look for pro-rata or full replacement policies after year two, and ensure the warranty is valid through authorized service centers globally. |
| 5. How do I ensure ongoing service and technical support after the warranty period expires? | Partner with manufacturers offering post-warranty service contracts, including annual preventive maintenance, firmware updates, and priority repair turnaround (typically ≤5 business days). Top-tier suppliers now provide IoT-enabled devices that monitor ozone output, usage cycles, and component health—enabling predictive servicing. Distributors should verify local technical support networks and access to certified biomedical engineers trained on ozone safety standards (e.g., OSHA and EU Directive 2014/34/EU). |
Need a Quote for Dental Ozone Therapy Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


