Article Contents
Strategic Sourcing: Dental Periapical X Ray Machine
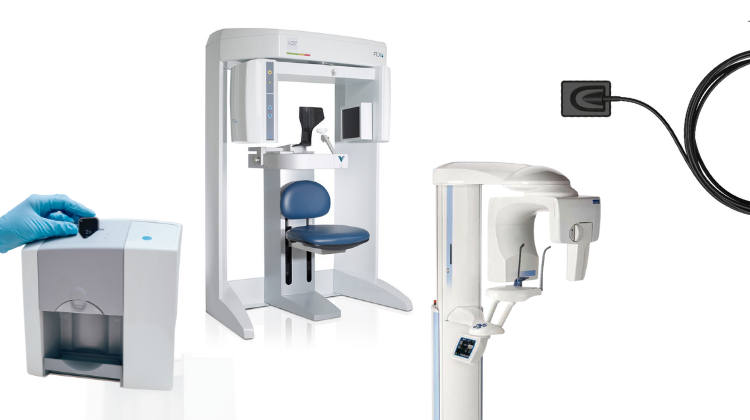
Executive Market Overview: Dental Periapical X-Ray Machines in Modern Digital Dentistry
Dental periapical X-ray machines remain indispensable cornerstones of evidence-based clinical diagnostics in contemporary dental practice. As digital dentistry accelerates toward integrated, precision-focused workflows, these systems have evolved beyond basic imaging tools to become critical components of diagnostic ecosystems. Their significance in 2026 stems from three pivotal functions: enabling precise endodontic diagnosis through sub-millimeter resolution of periapical pathologies, facilitating immediate treatment verification during surgical procedures, and generating DICOM-compliant datasets for AI-driven diagnostic support systems. With radiation doses reduced by 80-90% compared to film-based predecessors and seamless integration into practice management software (PMS) via HL7/FHIR protocols, modern digital periapical units directly support value-based care models by minimizing retakes, accelerating treatment cycles, and providing auditable diagnostic documentation.
The global market exhibits a strategic bifurcation between premium European manufacturers and value-engineered Asian alternatives. Established European brands (Dentsply Sirona, Planmeca, Vatech) dominate high-end clinics with engineering precision and ecosystem integration but command 35-50% price premiums. Conversely, Chinese manufacturers have closed the quality gap through ISO 13485-certified production, with Carejoy emerging as a clinically validated alternative that balances CE/FDA compliance with 40-60% cost efficiency. For distributors, this segment represents a high-margin opportunity (25-35% gross) with clinics increasingly adopting “tiered procurement” strategies—deploying premium units in specialty departments while utilizing cost-optimized systems for routine diagnostics.
Technical & Commercial Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy (Chinese Manufacturer) |
|---|---|---|
| Image Quality & Resolution | 18-22 lp/mm resolution; patented noise-reduction algorithms; dynamic range optimization for low-dose imaging | 16-18 lp/mm resolution; AI-enhanced contrast adjustment; meets ISO 10970:2020 standards for diagnostic equivalence |
| Price Range (Per Unit) | €28,000 – €42,000 (excluding VAT) | €11,500 – €16,800 (FOB China; +18% landed cost) |
| Warranty & Service | 3-year comprehensive warranty; 24/7 onsite support in EU (4h SLA); proprietary service network | 2-year warranty; 72h remote diagnostics; distributor-certified technician network (95% coverage in EU via partners) |
| Compliance & Certifications | IEC 60601-2-54:2020; MDR 2017/745; FDA 510(k); integrated cybersecurity certification | CE Mark (MDR compliant); FDA 510(k) cleared; ISO 13485:2016; RoHS 3 certified |
| Integration Capabilities | Native PMS integration (Dentrix, OpenDental); AI diagnostic modules; cloud DICOM archiving | HL7/DICOM 3.0 standard interface; API for major PMS; optional AI add-on modules |
| Total Cost of Ownership (5-Year) | €48,500 – €67,200 (incl. service contracts @12% annually) | €22,800 – €29,400 (incl. service @8% annually; 35% lower power consumption) |
| Clinical Adoption Trend | Preferred for specialty practices (endodontics, oral surgery); 68% market share in >€500k revenue clinics | Rapid uptake in multi-chair general practices; 42% CAGR in EU value segment (2023-2026) |
Strategic Recommendation: For clinics prioritizing specialty workflows and ecosystem integration, premium European brands remain optimal. However, Carejoy demonstrates compelling value for volume-driven practices seeking MDR-compliant imaging with 58% lower entry cost and clinically equivalent diagnostic output for routine periapical assessments. Distributors should position Carejoy as a strategic entry-point product to capture market share in the €15k-€20k segment while bundling service contracts for sustained revenue. The convergence of Chinese manufacturing quality with European regulatory rigor has fundamentally reshaped procurement economics—making periapical X-ray technology accessible without compromising diagnostic integrity in the digital dentistry era.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Periapical X-Ray Machine
Designed for dental clinics and distribution partners, this guide provides a comprehensive comparison between Standard and Advanced models of dental periapical X-ray units. These specifications reflect industry standards, regulatory compliance, and technological advancements for 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 65 kVp maximum output, 4 mA tube current, fixed anode X-ray tube. Operates on standard 110–120 V AC, 50/60 Hz. Power consumption: ~350 VA. Requires no external cooling system. | 70 kVp maximum output, 8 mA tube current, rotating anode X-ray tube with enhanced heat dissipation. Operates on 100–240 V AC, 50/60 Hz (auto-switching). Power consumption: ~500 VA. Integrated thermal protection and active cooling system. |
| Dimensions | Wall-mounted or floor stand: 38 cm (W) × 25 cm (D) × 45 cm (H). Weight: 8.5 kg. Arm reach: 60 cm horizontal, 40 cm vertical adjustment. Compact design for small operatory integration. | Motorized floor stand or ceiling suspension: 42 cm (W) × 30 cm (D) × 52 cm (H). Weight: 12.3 kg. Articulating arm with 90 cm horizontal reach, 55 cm vertical travel, and 360° rotation. Ergonomic positioning with memory presets. |
| Precision | Manual positioning with mechanical locks. Beam alignment accuracy ±3°. Collimated circular beam field (6 cm diameter at 20 cm focus-skin distance). Timer range: 0.05–1.5 seconds in 0.05 sec increments. | Digital targeting with laser-guided alignment and real-time sensor feedback. Beam alignment accuracy ±0.5°. Rectangular collimation (reducing patient dose by up to 60%). Timer range: 0.02–1.2 seconds in 0.01 sec increments with exposure optimization algorithms. |
| Material | Housing constructed from reinforced ABS polymer and powder-coated steel base. X-ray tube housed in lead-lined aluminum casing (2.0 mm Pb equivalent shielding). Non-slip rubberized joints and handles. | Full aluminum-magnesium alloy chassis with anti-microbial coating. Lead-composite shielding (2.5 mm Pb equivalent) with modular, service-friendly paneling. Sealed bearings and corrosion-resistant joints for high-traffic clinical environments. |
| Certification | Complies with IEC 60601-1, IEC 60601-2-54, FDA 510(k) cleared, CE Marked (Medical Device Regulation EU 2017/745), ISO 13485 certified manufacturing. Meets ADA and AERB safety standards. | Full compliance with IEC 60601-1-2 (EMC), IEC 60601-2-54 (4th Ed.), FDA 510(k) with dose optimization claims, CE Marked under MDR, Health Canada licensed, ISO 13485 and ISO 14971 (risk management). Includes DICOM compatibility certification and cybersecurity compliance (IEC 81001-5-1). |
Note: Specifications are subject to change based on regional regulatory requirements and technical updates. Always verify compliance with local health authorities prior to installation.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Periapical X-Ray Machines from China
Target Audience: Dental Clinic Procurement Teams & International Dental Equipment Distributors
Validity: January 2026 – December 2026 | Prepared By: Global Dental Equipment Advisory Group
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
| Verification Step | 2026 Critical Actions | Risk of Non-Verification |
|---|---|---|
| ISO 13485:2016 Certification | Request current certificate (issued within 12 months) with scope explicitly covering “Medical Electrical Equipment – Diagnostic Radiography”. Validate via iso.org or accredited body database. Confirm manufacturer name matches factory legal entity. | Invalidates CE claims; voids product liability coverage; triggers FDA import alerts |
| CE Technical Documentation | Demand full Technical File per MDR Annexes II & III, including: – Risk Management Report (ISO 14971:2019) – Clinical Evaluation Report (CER) – 2026-specific EMC/EMI test reports (IEC 60601-1-2:2021) – Radiation safety dossier (IEC 60601-2-54:2022) |
MDR non-compliance; product recall risk; distributor liability for defective devices |
| Factory Audit Trail | Require: – 3 years of ISO surveillance audit reports – MDR-designated Notified Body certificate number – Proof of post-market surveillance (PMS) system – 2026 Requirement: Cybersecurity vulnerability assessment (per MDCG 2019-16) |
Regulatory blacklisting; inability to register devices in EU/UK markets |
Step 2: Negotiating MOQ & Commercial Terms
| Parameter | Market Standard (2026) | Negotiation Strategy | Red Flags |
|---|---|---|---|
| Minimum Order Quantity (MOQ) | 8-12 units for standard models 20+ units for OEM customization |
Leverage volume tiers: – Tier 1: 8 units @ base price – Tier 2: 15+ units @ 4-6% discount – Tier 3: 30+ units @ 7-9% discount + free shipping Note: Request “mixed model” MOQ flexibility for periapical/CBCT bundles |
MOQ < 5 units (indicates trading company markup); MOQ > 25 without OEM justification |
| Payment Terms | 30% deposit, 70% against BL copy LC at sight (for new partners) |
Negotiate: – 15-20% deposit for first order – Escrow payment for orders >$50k – Net 30 days after 3 successful shipments Require bank references from supplier |
Full prepayment demanded; payment to personal accounts |
| Warranty & Support | 12 months standard Extended warranties available |
Mandate: – On-site technician training (2 days) – 24-month warranty for X-ray generator – Spare parts kit (5% of order value) – Remote diagnostics access |
Warranty voided for “unauthorized service”; no local service network |
Step 3: Shipping & Logistics (2026 Critical Path)
| Term | Cost Structure | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Factory price only • + Freight ($1,800-$2,500/20ft) • + Insurance (0.3% value) • + Destination charges |
• Buyer bears all risk after cargo passes ship rail • Customs clearance delays = your liability • Port congestion surcharges (common in Shanghai) |
Only for: – Experienced importers with freight forwarders – Orders > $75k where freight savings justify risk |
| DDP (Delivered Duty Paid) | • All-inclusive price • Covers: Freight, Insurance, Import Duty, VAT, Customs Clearance • Transparent final cost (no hidden fees) |
• Supplier bears all risk until delivery at your door • Guaranteed delivery timeline • Duty calculation errors = supplier liability |
STRONGLY RECOMMENDED for: – First-time importers – Orders < $50k – Markets with complex regulations (EU/UK) |
Trusted Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Assurance: ISO 13485:2016 certified (Certificate #CN-2025-13485) with MDR-compliant CE technical files for all imaging equipment. Notified Body: TÜV SÜD (NB 0123)
- MOQ Flexibility: 8-unit MOQ for standard periapical units (model CJ-PA2026); 15-unit MOQ for OEM. Offers mixed-model orders (e.g., 4 periapical + 2 CBCT = 6-unit credit).
- DDP Expertise: Direct port access from Shanghai Waigaoqiao Terminal (WGT). Provides DDP quotes to 45+ countries with duty calculation guarantees.
- Technical Validation: 19-year manufacturing history with in-house X-ray generator production (critical for radiation safety compliance). Factory audit reports available upon NDA.
Procurement Contact:
Email: [email protected]
WhatsApp: +86 15951276160
Address: Room 1208, Building 3, No. 666 Gucun Road, Baoshan District, Shanghai, China
Note: Request “2026 Periapical Compliance Package” including radiation test reports and MDR gap analysis.
Final Advisory: In 2026, prioritize suppliers with demonstrable MDR-compliant documentation and Shanghai port logistics capabilities. Avoid “one-stop trading companies” – verify factory ownership via China Customs Export Record (HS Code: 90221200). All periapical X-ray machines must include DICOM 3.0 compatibility and ALARA-compliant dose reduction features per IEC 60601-2-54:2022.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: Buying a Dental Periapical X-Ray Machine in 2026
As dental imaging technology advances, selecting the right periapical X-ray unit requires due diligence in performance, compliance, and long-term support. Below are five critical questions dental clinics and distributors should consider when procuring equipment in 2026.
| Question | Answer |
|---|---|
| 1. What input voltage requirements should I verify before purchasing a periapical X-ray machine for my clinic? | Most modern dental periapical X-ray units operate on standard 100–240V AC, 50/60 Hz, making them suitable for global use. However, clinics must confirm local voltage stability and grounding compliance. Units in regions with unstable power (e.g., parts of Africa, South Asia) should include built-in voltage regulators or surge protection. Always consult the technical datasheet and ensure compatibility with your facility’s electrical infrastructure to prevent equipment damage or safety hazards. |
| 2. Are spare parts readily available, and what is the average lead time for critical components? | Reputable manufacturers and distributors maintain regional spare parts hubs to ensure availability of key components—such as X-ray tubes, collimators, control boards, and position-indicating devices (PIDs)—within 5–10 business days. When procuring, verify the supplier’s spare parts inventory, local stocking policies, and whether parts are backward-compatible across model generations. Distributors should confirm multi-year parts availability to support long-term service contracts. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of wall-mounted or floor-standing periapical units typically requires a certified biomedical technician. The process includes electrical safety checks, wall anchoring (for mounted units), alignment verification, and DICOM/network integration if connected to a digital imaging system. Most suppliers offer white-glove installation services as part of the purchase agreement. Remote clinics should confirm if the distributor provides on-site setup or if remote-guided installation with video support is available. |
| 4. What is the standard warranty coverage for a dental periapical X-ray machine in 2026? | Leading manufacturers offer a minimum 2-year comprehensive warranty covering parts, labor, and X-ray tube performance. Extended warranties up to 5 years are available for purchase. The warranty should include defects in materials, electronic failures, and mechanical faults under normal use. Note: Damage from power surges, improper handling, or unauthorized repairs is typically excluded. Distributors must provide clear warranty terms in writing and support claim processing locally. |
| 5. How are firmware updates and regulatory compliance managed during the warranty period? | In 2026, many digital periapical units include embedded software for dose optimization, image calibration, and connectivity (e.g., DICOM, cloud PACS). Manufacturers provide free firmware updates during the warranty term to maintain compliance with evolving standards (e.g., IEC 60601-2-54, FDA 510(k), EU MDR). Distributors should ensure clinics receive update notifications and technical support for seamless implementation. Regulatory documentation must be available in local languages upon request. |
Need a Quote for Dental Periapical X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


