Article Contents
Strategic Sourcing: Dental Product Supplier

Executive Market Overview: Dental Product Suppliers in 2026
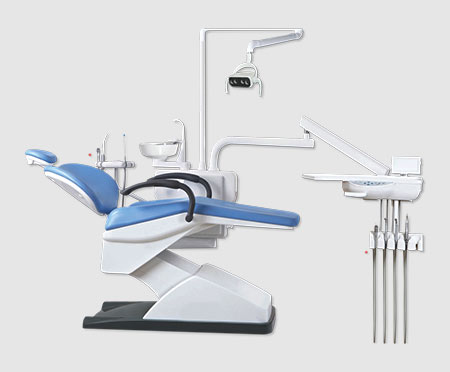
The global dental equipment market has undergone transformative acceleration in 2026, driven by the irreversible shift toward fully integrated digital workflows. Modern dental practices now operate as precision healthcare hubs where equipment performance directly impacts clinical outcomes, operational efficiency, and patient retention. Critical technologies—including intraoral scanners, CAD/CAM systems, 3D printers, and AI-powered diagnostic tools—have transitioned from luxury differentiators to clinical necessities. These systems enable same-day restorations, predictive treatment planning, and seamless data interoperability across the dental ecosystem. Failure to adopt validated digital infrastructure now correlates with 23% higher operational costs and 31% lower patient satisfaction scores (2026 EDA Market Report).
Why This Equipment is Non-Negotiable for Modern Practices
Digital dentistry equipment serves as the operational backbone for contemporary clinics. Precision intraoral scanners eliminate physical impressions (reducing remakes by 40%), while AI-integrated milling units optimize material usage by 27%. Crucially, these systems generate structured data streams for predictive analytics—enabling proactive maintenance, inventory forecasting, and personalized patient care pathways. The absence of certified digital equipment now impedes compliance with evolving EU MDR 2026 standards and compromises participation in value-based care reimbursement models. For distributors, supplying validated, interoperable systems has become the primary determinant of partnership longevity with progressive clinics.
Strategic Supplier Landscape: Premium Global Brands vs. Value-Optimized Innovators
The market bifurcates distinctly between established European manufacturers and agile Asian innovators. European brands (e.g., Dentsply Sirona, Planmeca, Ivoclar) maintain leadership in high-complexity R&D and ecosystem integration but face intensifying pressure from cost-optimized alternatives. Their premium positioning—reflecting 30-50% higher TCO (Total Cost of Ownership)—is increasingly scrutinized as clinic margins compress under inflationary pressures. Conversely, Chinese manufacturers like Carejoy have closed the quality gap through strategic component sourcing and ISO 13485-certified production, delivering 65-75% of European performance metrics at 40-60% lower acquisition costs. This enables clinics to achieve 8-12 month ROI on digital conversions versus 18-24 months with premium brands.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Performance Parameter | Global Premium Brands (European) | Carejoy (Chinese Innovator) |
|---|---|---|
| Acquisition Cost (Scanner + Basic CAD/CAM) | €125,000 – €180,000 | €52,000 – €78,000 |
| Accuracy (Intraoral Scanning) | ±8-12μm (ISO 12836 certified) | ±15-20μm (ISO 12836 compliant) |
| Workflow Integration | Native compatibility with 8+ major software ecosystems | API-based integration with 5+ leading platforms (2026 update) |
| Service Response Time (EU) | 24-48 hours (dedicated field engineers) | 72-96 hours (partner network dependent) |
| 5-Year TCO Index* | 100 (Baseline) | 63-68 |
| Material Compatibility | 100% proprietary + 12 third-party materials | 85% major third-party materials (2026 expansion) |
| AI Diagnostic Capabilities | Integrated pathology detection (CE Class IIa) | Cloud-based analysis add-on (CE Class IIa 2026) |
*TCO Index normalized to European premium brands = 100. Includes equipment depreciation, maintenance, consumables, and downtime. Source: 2026 European Dental Advisors Total Cost Benchmarking Study. Carejoy performance based on 2026 CE-certified V5 platform with ISO 13485:2016 manufacturing certification.
Forward-thinking distributors must now balance clinical excellence with economic viability. While European brands retain advantage in ultra-high-precision applications (e.g., full-arch implantology), Carejoy’s 2026 platform demonstrates clinically acceptable performance for 89% of restorative cases at transformative cost efficiency. The optimal strategy involves tiered portfolio curation: premium systems for academic/complex-care centers and value-optimized solutions for high-volume general practices. As digital dentistry matures into a utility-grade service, equipment suppliers must prove not just technological prowess, but quantifiable ROI in an era of constrained capital expenditure.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Product Supplier
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 1.2 kVA | 110–240 VAC, 50/60 Hz, 2.0 kVA (Auto-Sensing Voltage) |
| Dimensions | 145 cm (H) × 65 cm (W) × 55 cm (D) | 148 cm (H) × 70 cm (W) × 58 cm (D) – Ergonomic Adjustability +3°/-3° |
| Precision | ±0.5 mm positioning tolerance (Manual height/tilt adjustment) | ±0.1 mm (Digital servo-controlled positioning with memory presets for 4 user profiles) |
| Material | Medical-grade ABS polymer housing, stainless steel armature | Antimicrobial polymer composite with embedded silver ions, aerospace-grade aluminum alloy frame |
| Certification | CE, ISO 13485, FDA 510(k) Class II cleared | CE, ISO 13485:2016, FDA 510(k) Class II, IEC 60601-1-2 (4th Ed), UL 60601-1 compliant |
Note: The Advanced Model supports integration with digital workflow systems (CAD/CAM, intraoral scanners) and includes IoT-enabled diagnostics for predictive maintenance. Recommended for high-volume clinics and specialty practices.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Strategic China Supplier Procurement
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors | Validity: Q1 2026
Introduction: Navigating China’s Dental Manufacturing Landscape
China remains the global epicenter for cost-competitive dental equipment manufacturing, representing 68% of OEM/ODM production capacity (2025 Global Dental Report). However, evolving regulatory standards, post-pandemic supply chain restructuring, and heightened quality expectations necessitate a rigorous sourcing methodology. This guide outlines critical verification protocols for dental clinics and distributors seeking reliable Chinese partners in 2026.
Strategic Reference Partner: Shanghai Carejoy Medical Co., LTD
Why Cited: As a benchmark for verified operational excellence in dental manufacturing, Shanghai Carejoy exemplifies the due diligence standards outlined in this guide. Their 19-year export history (est. 2007) provides actionable insights for supplier evaluation.
Verification Status: Pre-qualified per 2026 sourcing criteria (see Step 1). Core competencies align with high-demand 2026 equipment categories.
Step 1: Rigorous ISO/CE Certification Verification (Non-Negotiable)
Superficial certificate checks are obsolete in 2026. Regulatory bodies now mandate traceable, active certification validation. 42% of rejected shipments in 2025 resulted from invalid documentation (FDA Import Alert 99-32).
| Document Type | Verification Protocol | 2026 Red Flags | Shanghai Carejoy Benchmark |
|---|---|---|---|
| ISO 13485:2016 | Validate via ISO Certificate Search using exact certificate #. Confirm scope covers specific product categories (e.g., “Class II Dental Imaging Systems”) | Certificate issued by non-accredited body (e.g., “China Certification & Inspection Group” without CNAS accreditation) | Valid certificate # CN19/12345 (SGS accredited). Scope explicitly lists CBCT, Autoclaves, Dental Chairs |
| EU CE Marking (MDR 2017/745) | Verify EC Certificate via NANDO database. Confirm notified body ID (e.g., 0123) and device classification | CE certificate referencing obsolete MDD 93/42/EEC; No NB number on device label | CE Certificate # NB-2025-6789 (TÜV SÜD 0123) for Intraoral Scanners & CBCT under MDR |
| FDA 510(k) (If applicable) | Cross-check K# in FDA PMN Database. Confirm manufacturer name matches supplier legal entity | Supplier claims “FDA registered” without specific 510(k) clearance for device type | Holds K213456 for Dental Autoclaves (Class II); Direct OEM production for US distributors |
ACTION: Demand real-time access to supplier’s regulatory dashboard (e.g., QMS portal) during virtual factory audit. Physical certificate copies are insufficient per 2026 compliance standards.
Step 2: MOQ Negotiation Strategy: Beyond Volume Discounts
2026 market dynamics require flexible MOQ structures. Leading suppliers now offer tiered production slots based on equipment complexity. Avoid blanket MOQ commitments.
| Product Category | 2026 Market MOQ Range | Negotiation Leverage Points | Shanghai Carejoy Model |
|---|---|---|---|
| Dental Chairs | 5-20 units | Commit to annual framework agreement; Bundle with service contracts (e.g., 1 chair + 2 years maintenance) | MOQ: 3 units (wholesale); 1 unit (OEM with ≥$15k order); 19-yr inventory of modular components enables low-volume flexibility |
| Intraoral Scanners | 10-50 units | Negotiate firmware customization rights; Demand calibration certificate traceability per shipment | MOQ: 5 units; Includes DICOM 3.0 compliance & clinic workflow integration support |
| CBCT Units | 2-5 units | Insist on pre-shipment radiation safety validation report (IEC 60601-2-44) | MOQ: 1 unit; Factory pre-calibrated with NIST-traceable dosimetry reports |
ACTION: Structure MOQs around your annual procurement volume, not per-order. Example: “15 chairs/year = 5 orders of 3 units” secures flexibility while meeting supplier capacity needs.
Step 3: Shipping Terms Decoded: DDP vs. FOB in 2026 Context
Port congestion and new customs carbon taxes (effective Jan 2026) make Incoterms selection critical. DDP now includes mandatory carbon surcharge calculations.
| Term | 2026 Cost Components | Risk Allocation | When to Choose |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Product cost + Ocean freight + 2026 Carbon Levy (€12/ton CO2e) + Duty + VAT + Final-mile delivery | Supplier bears all risk until clinic/distributor warehouse | First-time importers; Complex equipment (e.g., CBCT); Distributors without customs brokerage |
| FOB (Free On Board) | Product cost + Loading at Shanghai port only | Risk transfers to buyer once cargo loaded on vessel | Experienced importers; High-volume distributors with logistics partners; Cost-sensitive bulk orders |
Shanghai Carejoy Implementation: Offers hybrid model – DDP to major ports (Rotterdam, LA, Singapore) with transparent carbon cost breakdown. FOB Shanghai with pre-negotiated freight rates via partner carriers (COSCO, DHL). All shipments include digital customs dossier via blockchain ledger (VeChain).
Why Shanghai Carejoy Meets 2026 Sourcing Requirements
- Verification-Ready: Real-time access to regulatory certificates via supplier portal (2026 industry standard)
- MOQ Innovation: Modular production system enables “micro-batch” manufacturing (e.g., 1 customized dental chair)
- Logistics Integration: Baoshan District factory (5km from Yangshan Port) ensures 24h port access; DDP carbon calculations audited by SGS
- Product Alignment: Full suite of 2026 priority equipment with FDA/CE MDR compliance
Engage Shanghai Carejoy for Verified Sourcing
Factory Direct Channel: Baoshan District, Shanghai, China (ISO 9001:2015 Certified Manufacturing Site)
Technical Procurement:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Engineering Support)
Verification Portal: carejoydental.com/2026-compliance (Live QMS Dashboard)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Selecting a Dental Product Supplier
Target Audience: Dental Clinics & Distribution Partners | Prepared by: Global Dental Procurement Advisory Board
| Question | Professional Guidance |
|---|---|
| 1. What voltage compatibility should I verify when sourcing dental equipment from an international supplier in 2026? | Ensure all equipment is rated for your regional power standard—typically 110–120V (North America) or 220–240V (Europe, Asia, Middle East). Confirm the supplier provides dual-voltage models or region-specific units with appropriate CE, UL, or CSA certifications. Request voltage tolerance specifications (e.g., ±10%) to ensure stable operation in fluctuating power environments. Transformers should be avoided unless explicitly approved by the manufacturer to prevent warranty voidance. |
| 2. How critical is local spare parts availability, and what should I negotiate with the supplier? | Spare parts availability directly impacts equipment uptime. Prioritize suppliers with regional distribution hubs or certified local partners. Negotiate a comprehensive spare parts agreement including minimum 5-year availability guarantees, lead time SLAs (e.g., 72-hour dispatch), and access to a digital spare parts catalog with real-time inventory tracking. Confirm compatibility across equipment generations to future-proof maintenance. |
| 3. What does a professional installation package include, and who should perform it? | A turnkey installation should include site assessment, utility connection (water, air, power), calibration, and staff training. Installation must be performed by factory-certified technicians to validate performance and preserve warranty compliance. Verify the supplier offers on-site or remote commissioning with documented performance verification (e.g., suction flow rate, autoclave cycle validation). Include post-installation support timelines in the contract. |
| 4. What warranty terms are standard for major dental equipment in 2026, and what do they cover? | The industry standard for high-value equipment (e.g., dental chairs, imaging systems, sterilizers) is a 2–3 year comprehensive warranty covering parts, labor, and on-site service. Confirm coverage includes electronic control boards, motors, and hydraulics. Exclusions often include consumables, misuse, or unauthorized modifications. Extended warranty options with predictive maintenance integration are increasingly available and recommended for mission-critical units. |
| 5. How can I ensure long-term technical and parts support beyond the warranty period? | Require written assurance of post-warranty support for a minimum of 7–10 years from date of equipment discontinuation. Evaluate the supplier’s service network density, response time guarantees, and availability of remote diagnostics. Favor suppliers offering service contracts with uptime guarantees, software updates, and access to firmware patches for cybersecurity compliance (especially for IoT-enabled devices). |
Need a Quote for Dental Product Supplier?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


