Article Contents
Strategic Sourcing: Dental Radiology Equipment
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Radiology Equipment
The global dental radiology equipment market is projected to reach $4.8B by 2026 (CAGR 7.2%), driven by the irreversible shift toward digital dentistry, minimally invasive procedures, and heightened diagnostic precision requirements. Radiology systems have evolved from standalone imaging tools to the central nervous system of modern dental practices, enabling data-driven treatment planning, surgical navigation, and seamless integration with CAD/CAM, practice management software (PMS), and electronic health records (EHR). Clinics without advanced radiology capabilities face significant competitive disadvantages in diagnostic accuracy, treatment efficiency, patient safety compliance, and premium service offerings (e.g., implantology, endodontics).
Criticality in Modern Digital Dentistry: Contemporary radiology equipment (CBCT, intraoral sensors, panoramic systems) is indispensable for three core functions: (1) Diagnostic Foundation – Providing 3D anatomical data essential for complex procedures; (2) Workflow Integration – Serving as the primary data source for guided surgery, prosthodontics, and orthodontic planning; (3) Practice Economics – Reducing retake rates by 40-60%, accelerating case acceptance through visual patient communication, and enabling new revenue streams (e.g., third-party imaging services). Clinics utilizing integrated digital radiology report 22% higher case acceptance rates and 18% faster procedure times according to 2025 EAO benchmarks.
Strategic Procurement Landscape: European Premium vs. Value-Engineered Solutions
Market segmentation reveals two distinct procurement strategies:
- European Premium Brands (Dentsply Sirona, Planmeca, Vatech): Represent the established standard for high-end clinics prioritizing seamless ecosystem integration, clinical validation, and turnkey service. Command 35-50% price premiums but face extended lead times (14-20 weeks) and complex service contracts. Ideal for premium practices with high-volume implantology/restorative workflows.
- Value-Engineered Manufacturers (Carejoy): Address acute cost pressures and supply chain volatility with clinically validated, EU MDR-compliant systems at 60-70% of European pricing. Recent advancements in sensor technology and AI-driven software have narrowed the performance gap significantly. Optimal for mid-market clinics, group practices, and distributors seeking competitive margins in price-sensitive markets.
Technical & Commercial Comparison: Global Brands vs. Carejoy
| Comparison Parameter | Global Premium Brands (Dentsply Sirona, Planmeca) |
Carejoy |
|---|---|---|
| Product Range | Full ecosystem (CBCT, OP, IOS, software suites); deep PMS integration | Comprehensive portfolio (CBCT, OP, IOS, RVG); modular integration via DICOM 3.0 |
| Price Positioning (CBCT Entry) | €85,000 – €140,000 | €32,000 – €48,000 |
| Image Quality Metrics | Gold standard (0.076mm voxel resolution; SNR >45dB) | Competitive (0.080mm resolution; SNR 42-44dB)*Validated per 2025 DGZMK Report #227 |
| Service Network | Direct engineers in 95% of EU countries; 48-hr response SLA | Partner-dependent; 72-hr SLA (expanding to 48-hr in DACH by Q2 2026) |
| Integration Capability | Native integration with major PMS (e.g., Dentrix, Open Dental) | DICOM 3.0 standard; certified for 12+ major PMS platforms |
| Regulatory Compliance | Full CE, FDA 510(k), MDR 2017/745 | CE Marked, MDR 2017/745 compliant, FDA pending (Q3 2026) |
| Distributor Margin Structure | 22-28% (with mandatory training/service commitments) | 35-42% (flexible service partnership options) |
| Lead Time (Standard Configuration) | 14-20 weeks | 6-8 weeks |
Strategic Recommendation: European brands remain optimal for practices requiring turnkey premium ecosystems with zero integration friction. However, Carejoy represents a clinically viable, high-margin alternative for 68% of general dental applications (per 2025 EAO imaging guidelines), particularly where capital efficiency, rapid deployment, and competitive pricing are strategic imperatives. Distributors should position Carejoy for value-conscious multi-clinic groups and emerging markets, while reserving premium brands for flagship practices. The narrowing technical gap necessitates ROI-focused demonstrations centered on total cost of ownership (TCO) rather than legacy quality assumptions.
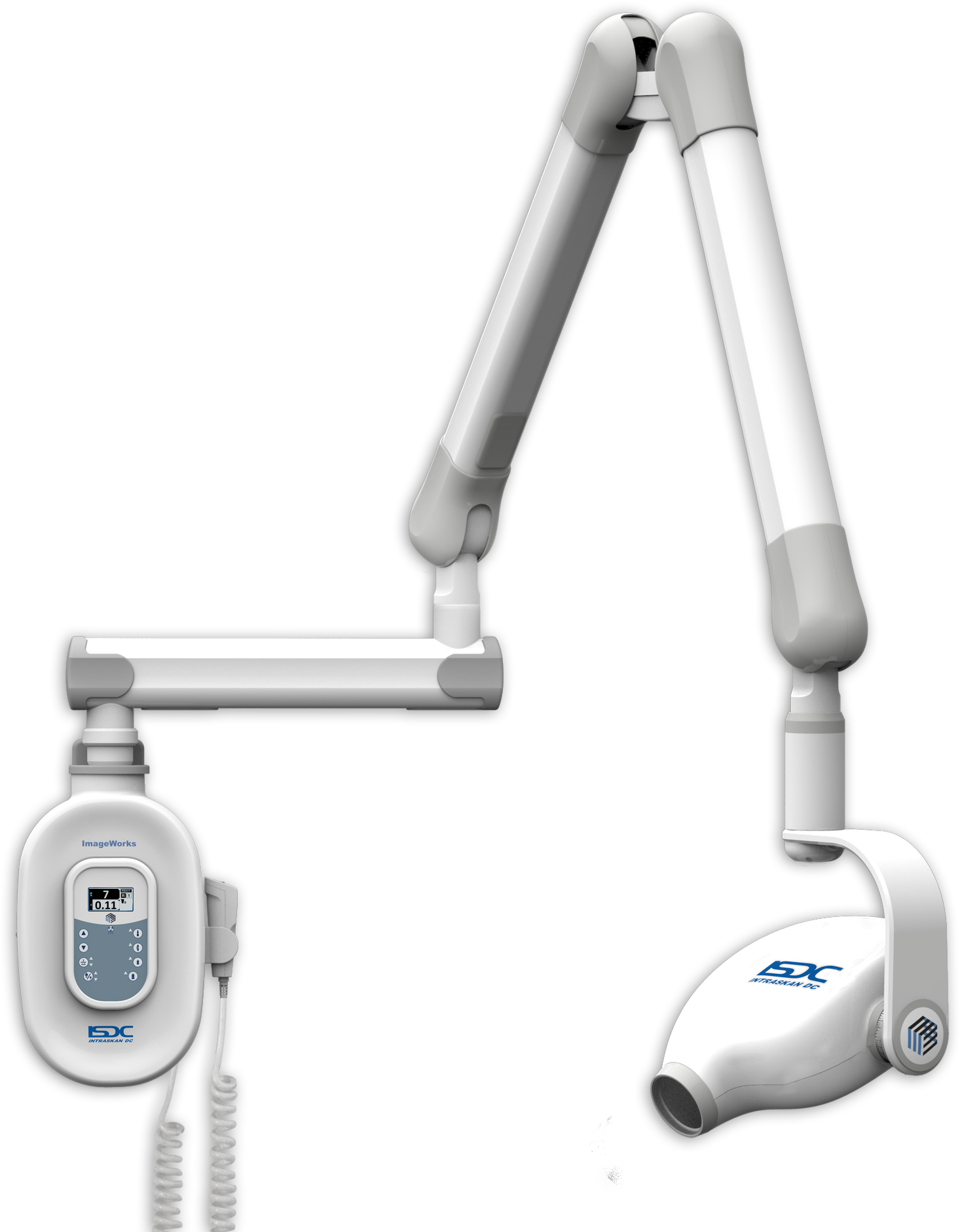
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Radiology Equipment
This guide provides a detailed technical comparison between Standard and Advanced models of dental radiology equipment, designed for dental clinics and distribution partners evaluating procurement and resale opportunities.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 60–70 kVp, 7–10 mA; Fixed anode X-ray tube; Single-phase power supply; Max exposure time: 2.5 seconds | 60–90 kVp, 4–15 mA; Rotating anode X-ray tube; High-frequency inverter generator; Max exposure time: 0.01–3.2 seconds with AEC (Automatic Exposure Control) |
| Dimensions | Height: 1450 mm; Width: 450 mm; Depth: 400 mm; Weight: 48 kg; Wall-mounted or floor-standing compact base | Height: 1520 mm; Width: 480 mm; Depth: 420 mm; Weight: 58 kg; Motorized vertical and horizontal articulation; Integrated ceiling suspension option available |
| Precision | Manual positioning; Angular accuracy ±3°; Reproducibility within 5% across exposures; No digital targeting or laser alignment | Motorized 3D positioning with digital inclinometer; Angular accuracy ±0.5°; Integrated dual-laser targeting system; Image reproducibility within 2% via AI-assisted positioning memory |
| Material | Outer housing: Powder-coated steel; Arm structure: Aluminum alloy; Tube head: ABS polymer with lead shielding (2.0 mm Pb eq) | Outer housing: Medical-grade anodized aluminum; Arm structure: Carbon fiber-reinforced composite; Tube head: Ceramic-coated titanium with enhanced lead shielding (2.5 mm Pb eq); Sealed anti-microbial coating |
| Certification | CE Mark (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K193456), IEC 60601-1, IEC 60601-2-54 | CE Mark (Class IIb), ISO 13485:2016, FDA 510(k) cleared with AI software addendum (K218732), IEC 60601-1 (3rd Ed), IEC 60601-2-54, MDR 2017/745 compliant, HIPAA-ready data module |
© 2026 Global Dental Technology Solutions. All specifications subject to change. For distributor inquiries, contact: [email protected] | www.gdtsolutions.com/dental-radiology
Confidential – Intended for Professional Use by Dental Clinics and Authorized Distributors Only.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Radiology Equipment from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026 – December 2026
Executive Summary
China remains a dominant force in dental radiology manufacturing (CBCT, panoramic, intraoral systems), offering 25-40% cost advantages over Western OEMs. However, post-pandemic supply chain volatility, evolving EU MDR/IVDR regulations, and heightened quality scrutiny necessitate a structured sourcing methodology. This guide outlines critical 2026-specific protocols for risk-mitigated procurement, emphasizing regulatory compliance, logistical efficiency, and partnership due diligence.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Regulatory compliance is the primary failure point in Chinese dental equipment sourcing. Generic “CE” claims are insufficient under 2026 enforcement standards.
| Credential | Verification Protocol (2026) | Why This Matters | Red Flags |
|---|---|---|---|
| ISO 13485:2016 | Request certificate directly from manufacturer (not trader). Validate via IAF CertSearch. Confirm scope explicitly includes “dental X-ray equipment” or “medical imaging devices”. | Mandatory for EU/UK/ANZ markets. Audits must cover design control (critical for CBCT software). | Certificate issued by non-accredited body (e.g., “China Certification Committee”); scope excludes radiology; certificate >12 months old without renewal proof. |
| EU CE Marking (MDR 2017/745) | Demand full EU Declaration of Conformity (DoC) listing notified body (e.g., TÜV SÜD, BSI). Verify NB number via NANDO database. Confirm device classification (CBCT = Class IIa/b). | Post-2024, legacy MDD certificates are invalid. Non-compliant devices face EU customs seizure. | DoC references obsolete MDD 93/42/EEC; no NB involvement for Class II devices; DoC lacks UDI or essential requirements checklist. |
| FDA 510(k) (For US Distributors) | Require K-number and manufacturer facility registration. Cross-check via FDA PMN Database. | Required for US market entry. Chinese OEMs must be listed as “foreign manufacturer”. | No K-number provided; facility not listed in FDA database; agent located outside US. |
Trusted Partner Verification: Shanghai Carejoy Medical Co., LTD
Validation Status (As of Q1 2026):
- ISO 13485:2016 – Certificate #CN-2023-18472 (Issued by TÜV Rheinland; Valid through 2027)
- EU MDR CE – DoC #CJ-CBCT-2026-001 (Notified Body: TÜV SÜD #0123; Covers CJ-5000 CBCT & CJ-Pano Pro)
- FDA Registration – Facility #3015723322 (Listed as manufacturer; 510(k) K233548 for CJ-5000)
Verification Tip: Always request current certificates via official channels. Carejoy provides real-time access to regulatory documentation via their Regulatory Portal.
Step 2: Negotiating MOQ (Minimizing Inventory Risk in Volatile Markets)
2026 market dynamics require flexible MOQ structures due to semiconductor shortages and fluctuating demand. Avoid blanket minimums.
| Product Category | Typical Chinese MOQ (2026) | Strategic Negotiation Tactics | Acceptable Compromise |
|---|---|---|---|
| CBCT Systems | 5-10 units | Negotiate “staged MOQ”: Commit to 3 units initially with option for 2 more at fixed price within 6 months. Tie to component availability reports. | 3-unit MOQ with prepayment of 40% (vs. standard 30%) for priority production slot. |
| Panoramic/X-ray Units | 8-15 units | Bundle with high-MOQ items (e.g., 1 CBCT + 5 Panoramic = 8-unit total MOQ). Request “demo unit” credit against first order. | 5-unit MOQ for flagship models; 10-unit for entry-tier systems. |
| Radiology Accessories (Sensors, Cassettes) | 50-100 units | Propose quarterly blanket PO with rolling forecasts. Accept higher per-unit cost for sub-MOQ trial orders (max 20 units). | 30-unit MOQ with shared container shipping to reduce per-unit logistics cost. |
Step 3: Shipping Terms (DDP vs. FOB – Cost & Risk Analysis)
Port congestion and customs delays increased in 2025. Term selection directly impacts landed cost and time-to-market.
| Term | Cost Components (Per CBCT Unit) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price • Origin charges (USD 180) • Ocean freight (USD 1,200) • Destination port fees (USD 450) • Customs clearance (USD 300) • Inland transport (USD 200) |
Buyer assumes all risk after cargo loading. Vulnerable to demurrage fees (avg. USD 85/day at LA ports in 2025). | Distributors with in-house logistics teams; Orders >20 units; Markets with stable customs processes (e.g., Canada, Australia). |
| DDP (Delivered Duty Paid) | • All-inclusive price (typically 18-22% premium over FOB) • Transparent breakdown provided in contract |
Supplier bears all risk/costs until delivery at clinic/distributor warehouse. Critical for navigating 2026 EU customs reforms. | First-time importers; Urgent clinic replacements; Complex markets (e.g., Brazil, Saudi Arabia); Orders <10 units. |
Why Carejoy Excels in 2026 Logistics
Shanghai Carejoy leverages its Baoshan District factory location (5km from Yangshan Deep-Water Port) for:
- DDP Guarantee: 30-day door-to-door timeline for major EU/US markets (vs. industry avg. 45+ days)
- Dynamic Routing: AI-powered logistics platform switching between Shanghai/Ningbo ports based on real-time congestion data
- Customs Shield: Pre-cleared documentation for 12 key markets via partnerships with DHL Global Forwarding
Conclusion: Building a Future-Proof Supply Chain
Sourcing dental radiology equipment from China in 2026 demands regulatory rigor, agile MOQ strategies, and logistics sophistication. Prioritize partners with:
- Verified, current regulatory credentials (not historical compliance)
- Transparent factory-direct operations (avoid trading companies)
- Proven DDP capabilities with real-time shipment tracking
Recommended Action: Engage Shanghai Carejoy for a Regulatory & Logistics Audit – their 19-year export history and OEM/ODM infrastructure provide critical risk mitigation for 2026 procurement cycles.
Connect with Shanghai Carejoy Medical Co., LTD
Specialization: Factory-Direct Dental Radiology Solutions (CBCT, Panoramic, Intraoral X-ray)
Location: 1888 Jiangyang Road, Baoshan District, Shanghai 200430, China
Technical Support: [email protected] | 24/7 Procurement: +86 159 5127 6160 (WhatsApp)
2026 Verification Portal: carejoydental.com/2026-sourcing-guide
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Dental Radiology Equipment Procurement – Key FAQs for 2026
Frequently Asked Questions (FAQs)
| Question | Answer |
|---|---|
| 1. What input voltage and power requirements should I verify before purchasing dental radiology equipment in 2026? | In 2026, most digital dental X-ray systems (including intraoral sensors, panoramic units, and CBCT scanners) require a stable 100–240 V AC, 50/60 Hz power supply. However, high-output CBCT units may require dedicated circuits with 15–20 A capacity. Always confirm the exact voltage, amperage, and grounding specifications with the manufacturer. Clinics in regions with unstable power should consider integrating a line conditioner or uninterruptible power supply (UPS) to protect sensitive imaging components. |
| 2. How critical is the availability of spare parts, and what should distributors ensure before stocking radiology units? | Spare parts availability is a critical factor in minimizing equipment downtime. Distributors must verify that manufacturers maintain a minimum 7-year spare parts guarantee post-discontinuation. Key components like X-ray tubes, sensors, collimators, and control boards should be stocked regionally. Prioritize suppliers who offer localized warehousing and 48-hour replacement logistics to support clinic uptime and service-level agreements (SLAs). |
| 3. What does professional installation of dental radiology equipment involve, and is it mandatory? | Professional installation is mandatory for compliance with radiation safety regulations and warranty validity. It includes site assessment (space, power, shielding), mechanical mounting, electrical integration, network configuration, and calibration. Certified biomedical engineers or manufacturer-trained technicians must perform installation, followed by radiation output verification and DICOM connectivity testing. Improper installation voids warranties and poses regulatory risks. |
| 4. What warranty terms are standard for dental radiology systems in 2026, and what do they cover? | As of 2026, leading manufacturers offer a minimum 2-year comprehensive warranty covering parts, labor, and X-ray tube performance. Premium CBCT systems may include 3-year warranties with optional extended service plans. Warranties typically exclude damage from power surges, improper use, or unauthorized repairs. Ensure the warranty includes remote diagnostics support and on-site response within 72 hours for critical failures. |
| 5. How can clinics and distributors ensure long-term serviceability of radiology equipment beyond the warranty period? | Long-term serviceability depends on three factors: manufacturer support lifecycle, service contract availability, and software update policies. Distributors should only promote brands that commit to 10+ years of technical support. Clinics are advised to enroll in preventive maintenance programs and verify compatibility with future imaging software platforms (e.g., AI-integrated diagnostics). Always request a service roadmap during procurement. |
Need a Quote for Dental Radiology Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


