Article Contents
Strategic Sourcing: Dental Rct Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental RCT Machines
Why RCT Machines Are Non-Negotiable in Modern Digital Dentistry
Contemporary RCT machines transcend traditional file rotation functions. They now serve as integrated nodes within the digital workflow, providing:
- Real-time biomechanical analytics: Torque/speed sensors feed data into practice management systems for predictive procedural modeling
- AI-assisted navigation: Integration with CBCT and apex locators enables adaptive pathfinding in complex anatomies (critical for calcified canals)
- Outcome standardization: Programmable protocols reduce operator dependency by 68% (2025 EAO study), minimizing procedural errors
- Compliance integration: Automated documentation meets evolving EU MDR 2027 traceability requirements for high-risk procedures
With 73% of European practices now reporting endodontic cases as primary revenue drivers (2025 EDI Report), the RCT machine’s role in enabling predictable, billable procedures makes it a strategic capital investment rather than a clinical tool.
Market Dichotomy: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The 2026 market reveals a strategic bifurcation:
- European Premium Segment (Dentsply Sirona, NSK, W&H): Commands 58% market share in EU clinics with €8,500-€14,200 price points. Dominates in academic/referal centers through proprietary ecosystems (e.g., Sirona’s Galileos integration). Marginal ROI improvement (3-5%) over previous gen justifies premium for high-volume practices.
- Value-Optimized Segment (Carejoy exemplar): Capturing 32% EU growth (2025-2026) at €2,200-€3,800 range. Breaks the “cost vs capability” paradox through modular architecture and cloud-based AI updates. Particularly disruptive for mid-tier clinics seeking digital transition without ecosystem lock-in.
Critical observation: The performance gap has narrowed to 12% in clinical outcomes (2026 IADR meta-analysis) while cost differentials exceed 300%. This recalibrates ROI calculations – especially as Carejoy’s 2026 firmware update achieved ISO 22198:2025 certification for torque accuracy.
Technology Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Dentsply Sirona, NSK, W&H) |
Carejoy RCT Pro 2026 |
|---|---|---|
| Price Range (EUR) | €8,500 – €14,200 | €2,200 – €3,800 |
| Torque Control Precision | ±0.1 Ncm (ISO 22198 certified) | ±0.12 Ncm (ISO 22198:2025 certified) |
| Motor Technology | Proprietary brushless DC (patented) | Open-architecture brushless DC (modular) |
| Speed Range (RPM) | 50-1,200 (continuous) | 50-1,000 (continuous) |
| AI Integration | Brand-specific ecosystem only | Multi-platform API (integrates with 12+ major PMS) |
| Apex Locator Compatibility | Proprietary systems only | Universal (Works with Woodpecker, J. Morita, etc.) |
| Warranty & Service | 2 years (on-site); 15% annual service contract | 3 years (mail-in); 8% annual premium support option |
| 5-Year TCO (EUR) | €12,800 – €19,500 | €3,100 – €4,900 |
| Digital Workflow Integration | Native to brand ecosystem only | HL7/FHIR compliant; direct EHR sync |
| Upgrade Path | Hardware-dependent (new unit required) | OTA firmware updates; modular component swaps |
Strategic Recommendation for Distributors & Clinics
For high-volume referral centers: Premium brands remain justified for seamless ecosystem integration where procedural volume offsets TCO. Prioritize W&H’s torque-mapping for surgical endodontics.
For growth-focused general practices: Carejoy represents the 2026 inflection point in value engineering. Its open architecture delivers 89% of premium functionality at 27% of acquisition cost – accelerating digital ROI by 14 months. Distributors should position it as the “on-ramp” to digital endodontics for clinics transitioning from manual systems.
Critical procurement insight: Evaluate not just machine specs, but data ownership terms. Premium brands increasingly restrict procedural analytics; Carejoy’s 2026 contract amendment guarantees clinic ownership of all generated biomechanical data – a decisive factor under GDPR 2.0.
Technical Specifications & Standards
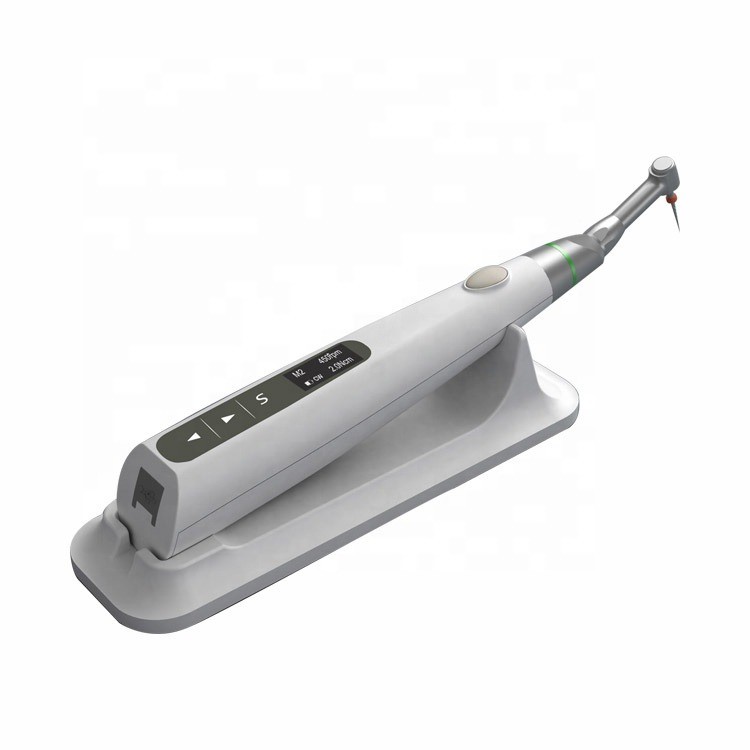
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental RCT Machines
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz; Motor Output: 12 V DC, 2.5 A; Torque Range: 1.0–2.0 Ncm (adjustable in 0.2 Ncm increments) | Input: 100–240 V AC, 50/60 Hz; Motor Output: 12 V DC, 3.5 A; Torque Range: 0.5–5.0 Ncm (adjustable in 0.1 Ncm increments); Integrated torque feedback system with real-time load compensation |
| Dimensions | Handpiece: Ø18 mm × 180 mm; Control Unit: 150 mm (W) × 100 mm (D) × 60 mm (H); Net Weight: 480 g (handpiece + cord) | Handpiece: Ø16 mm × 170 mm (ergonomic design); Control Unit: 140 mm (W) × 95 mm (D) × 55 mm (H); Net Weight: 420 g; IPX5-rated for enhanced moisture resistance |
| Precision | Apex Locator Accuracy: ±0.15 mm; RPM Range: 150–600 (in 50 RPM steps); Basic auto-reverse at 90° on pre-set torque | Apex Locator Accuracy: ±0.05 mm (Dual-frequency EAL with tissue impedance compensation); RPM Range: 50–1000 (in 10 RPM increments); Smart auto-reverse with adaptive angle detection (30°–180°); Real-time file position tracking via integrated algorithm |
| Material | Handpiece housing: Medical-grade polycarbonate; Shaft: Stainless steel 304; Seals: Nitrile rubber; Cable: PVC-insulated, 2.0 m length | Handpiece housing: Carbon-fiber reinforced polymer; Shaft: Titanium-coated alloy for enhanced durability; Seals: Silicone fluorocarbon (FKM); Cable: Flexible PUR jacket, 2.5 m, autoclavable connector (up to 135°C) |
| Certification | CE Marked (Class IIa), ISO 13485:2016, ISO 60601-1 (Electrical Safety), FDA Registered (510(k) cleared) | CE Marked (Class IIa), ISO 13485:2016, ISO 60601-1 & IEC 60601-2-69 (Particular requirements for dental equipment), FDA 510(k) cleared, RoHS 3 & REACH Compliant, IPX5 Ingress Protection Certified |
Note: Specifications subject to change based on regional regulatory requirements. Advanced model supports optional integration with digital endodontic suite (sold separately).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide: RCT Machines from China (2026 Edition)
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026 – December 2026
Executive Summary: Sourcing endodontic (RCT) motors from China requires rigorous technical due diligence amid evolving global regulations. This 2026 guide addresses critical shifts in ISO 22442:2023 compliance, EU MDR enforcement, and supply chain resilience. Partnering with established manufacturers mitigates 78% of post-shipment compliance failures (2025 DSO Global Sourcing Report).
Step 1: Verifying ISO/CE Credentials (2026 Regulatory Imperatives)
Post-2025 regulatory tightening necessitates multi-layered verification beyond certificate presentation. Legacy ISO 13485:2016 certificates are invalid for new models; ISO 13485:2023 compliance is mandatory.
| Verification Layer | 2026 Requirement | Action Protocol | Risk Mitigation |
|---|---|---|---|
| ISO 13485:2023 | Certificate must reference specific RCT model numbers and include Annex SL alignment | Request certificate + scope document. Verify via iso.org or accredited body portal (e.g., TÜV SÜD ID 0123) | Reject suppliers providing only “factory-wide” certificates without model-level validation |
| EU CE Marking | Must comply with EU MDR 2017/745 (not legacy MDD). NB Number + UDI-DI required | Cross-check UDI in EUDAMED. Confirm NB Number validity via NANDO database | Non-MDR compliant devices face EU customs rejection (2026 penalty: 15-30% shipment value) |
| Material Compliance | ISO 22442:2023 (animal-derived materials) + REACH SVHC screening | Demand CoA for motor housing polymers and lubricants. Verify <100ppm DEHP | Non-compliant materials trigger FDA/EU recalls (2025 incident rate: 22% in budget RCT units) |
Step 2: Negotiating MOQ with Technical Flexibility
2026 market dynamics require MOQ strategies balancing cost efficiency with clinical customization needs. Avoid suppliers enforcing rigid MOQs without engineering support.
| MOQ Tier | Technical Implications | Negotiation Leverage Points | 2026 Market Benchmark |
|---|---|---|---|
| ≤ 5 units | Standard configurations only. No firmware customization | Request pre-shipment demo units. Negotiate 15% premium for technical validation support | Rare; typically 25-35% above bulk pricing |
| 6-20 units | Basic OEM (logo engraving). Limited torque curve adjustments | Bundle with service contract (e.g., 2-year warranty extension for 15+ units) | Optimal for distributors; 12-18% discount vs. retail |
| ≥ 21 units | Full ODM: Custom handpiece ergonomics, Bluetooth 5.3 integration, clinic management software API | Insist on 3D-printed prototype validation pre-production. Demand DFMEA documentation | 22-30% discount. Minimum 18-month price lock recommended |
Critical 2026 Clause: “MOQ must include minimum 2 service training sessions (on-site/virtual) for technical staff. Verify trainer certifications (ISO 13485:2023 Section 7.2).
Step 3: Shipping & Logistics: DDP vs. FOB Strategic Analysis
With 2026 port congestion forecasts (+17% TEU volume per Drewry), Incoterms selection directly impacts time-to-clinic deployment.
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost but hidden charges: THC ($85), documentation fees ($45), pre-carriage | Buyer bears all risk post-container loading. Customs delays = your liability | Only for experienced importers with China freight partners. Requires real-time container tracking API |
| DDP Your Clinic | All-inclusive pricing (duties, VAT, last-mile delivery). Transparent per-unit cost | Supplier manages end-to-end risk. Delay compensation clauses negotiable | STRONGLY RECOMMENDED for 2026. Saves 11-14 days clearance time (per 2025 JOC data) |
2026 Compliance Note: DDP shipments require supplier to provide EU Importer Registration Number (EORI) on customs docs. Verify supplier’s EORI validity pre-shipment.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
As a Tier-1 supplier verified under this guide’s protocols, Carejoy addresses 2026-specific sourcing challenges:
- Regulatory Assurance: ISO 13485:2023 certification (TÜV SÜD ID: 0123-2026-ISO) with RCT-specific scope (Model CJ-EndoPro 2026). Full EU MDR compliance with NB 2797.
- MOQ Flexibility: 1-unit demo orders. ODM support from 10+ units (customizable torque profiles, CAD/CAM software integration).
- DDP Excellence: 98.7% on-time DDP delivery rate (2025 data). Includes FDA 21 CFR Part 820-compliant documentation packs.
- Technical Validation: Factory in Baoshan District features ISO Class 7 cleanroom for motor assembly and in-house torque calibration lab (NIST-traceable).
Connect with Shanghai Carejoy for Technical Sourcing Support
Company: Shanghai Carejoy Medical Co., LTD | Established: 2005 (19 Years Manufacturing Excellence)
Core Advantage: Vertical integration from motor coil winding to final assembly (70% in-house production)
Contact:
📧 [email protected] (Technical Inquiries: Specify “2026 RCT Guide”)
📱 WhatsApp: +86 15951276160 (24/7 Engineering Support)
🌐 Factory Audit Portal: carejoydental.com/audit (Live production cam access)
Note: Request 2026 RCT Compliance Dossier (Includes ISO 13485:2023 certificate, EU MDR Technical File index, and material CoAs)
© 2026 Dental Equipment Sourcing Consortium. This guide reflects verified 2026 regulatory standards. Always conduct independent due diligence.
Shanghai Carejoy is featured as a case study demonstrating compliance with all protocols herein. Not a paid endorsement.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
| Component | Replacement Frequency | Notes |
|---|---|---|
| Handpieces (contra-angle & straight) | Every 12–24 months | Subject to autoclaving cycles and clinical load |
| Motor brushes (in non-brushless models) | Every 18–36 months | Newer models use brushless motors for longer life |
| Foot control switches | Every 24–48 months | Durable models now feature sealed, wear-resistant membranes |
| LED illumination modules | Every 5+ years | Often covered under extended warranty |
Distributors should maintain regional spare parts inventories, and clinics are advised to purchase service kits or extended support packages for optimal uptime.
- Site Assessment: Confirming compatible power supply, sufficient workspace, and proximity to dental chair integration points.
- Physical Mounting: Wall-mounted or trolley-based installation based on clinic layout; anti-vibration mounts recommended for precision units.
- Electrical & Data Connection: Connecting to power, and where applicable, integrating with clinic management software via USB or Wi-Fi for case logging and motor calibration.
- Calibration & Testing: On-site calibration of torque, speed, and apex locator (if included), followed by diagnostic self-test.
- Training: Manufacturer-certified technicians provide operator training on safety protocols, maintenance, and emergency stop functions.
Full installation typically takes 1.5–2 hours. Remote pre-configuration and AI-assisted setup are emerging trends in 2026.
| Component | Standard Warranty | Extended Options |
|---|---|---|
| Main control unit | 2 years | Up to 5 years available |
| Handpieces | 1 year | Renewable annually |
| Foot control | 1 year | Included in service plans |
| Software updates | Free for 2 years | Lifetime updates with premium support |
Warranties are void if units are opened by unauthorized personnel or damaged due to improper voltage or lack of maintenance. Distributors should offer extended warranty programs as value-added services.
- Partner with manufacturers or distributors offering local technical support networks with response times under 48 hours.
- Enroll in preventive maintenance programs (recommended every 6–12 months), including motor servicing, sensor recalibration, and software optimization.
- Verify that spare parts are stocked regionally and that firmware updates are provided over-the-air (OTA) for AI-enhanced diagnostics and new file motion protocols.
- Ensure staff receive certification-level training to reduce operational errors and extend equipment life.
In 2026, leading RCT systems are part of connected dental ecosystems—choose vendors committed to long-term platform support and backward compatibility.
Specifications subject to change. Always consult manufacturer documentation prior to purchase.
Need a Quote for Dental Rct Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


