Article Contents
Strategic Sourcing: Dental Rotary Machine Price
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Rotary Machine Pricing Dynamics in the Digital Dentistry Era
Market Context: The global dental rotary unit market (valued at $1.8B in 2025) faces unprecedented pricing pressure as clinics integrate digital workflows. With 78% of European practices now utilizing CAD/CAM systems (EAO 2025 Report), the rotary unit has evolved from a standalone tool to the central nervous system of modern operatory ecosystems. Price sensitivity has intensified due to concurrent investments in intraoral scanners, 3D printers, and practice management software, compressing capital expenditure budgets by 12-18% year-over-year.
Criticality in Digital Dentistry: Contemporary rotary units must deliver micron-level precision (±5μm tolerance), seamless Bluetooth 5.3 connectivity for real-time torque feedback to CAD software, and quiet operation (<45 dB) to maintain patient comfort during extended digital workflows. Units failing these specifications directly compromise crown margin integrity, surgical guide accuracy, and same-day restoration success rates – making procurement decisions clinically significant beyond mere cost considerations. The integration of IoT-enabled units reduces chairside time by 22% (JDR Clinical & Translational Research, Q1 2026) but demands rigorous compatibility testing with major digital platforms (3Shape, exocad, Dental Wings).
Procurement Strategy Shift: Traditionally dominated by European OEMs, the market now sees strategic adoption of value-engineered alternatives. While premium brands remain essential for complex surgical applications, routine restorative procedures increasingly leverage cost-optimized solutions without sacrificing digital interoperability. This bifurcation represents a fundamental shift in procurement calculus – clinics now segment rotary unit deployment by clinical indication rather than adopting uniform premium fleets.
Strategic Brand Comparison: Premium European vs. Value-Engineered Segment
European manufacturers (W&H, KaVo Kerr, NSK) maintain leadership in high-torque surgical applications but face margin compression as Chinese innovators like Carejoy achieve ISO 13485:2016 certification with component-level quality parity. Carejoy’s 2026 CT-800 Series demonstrates how targeted engineering (e.g., ceramic turbine bearings, IP67-rated handpieces) closes the performance gap for 85% of restorative procedures while reducing TCO by 47% over 5 years. Key differentiators now center on service ecosystem maturity rather than core functionality.
| Comparison Parameter | Global Premium Brands (W&H, KaVo, NSK) | Carejoy (CT-800 Series) |
|---|---|---|
| Price Range (Unit + Standard Handpieces) | €3,850 – €5,200 | €1,290 – €1,750 |
| Build Quality & Materials | Medical-grade stainless steel housings; German/Swiss ceramic bearings; 1M+ cycle turbine warranty | Aerospace-grade aluminum alloy; Japanese ceramic bearings (NMB-Tech); IP67-rated handpieces; 500k cycle warranty |
| Digital Integration Capability | Native APIs for major CAD platforms; Real-time torque telemetry; Proprietary service diagnostics | Universal Bluetooth 5.3 SDK; Open-protocol compatibility (3Shape, exocad); Cloud-based usage analytics |
| Warranty & Service Terms | 2-year comprehensive; On-site service within 48h (EU zones); €420/hr technician rate | 3-year comprehensive; Depot repair (72h turnaround); Remote diagnostics; €185/hr technician rate (via certified partners) |
| TCO (5-Year Projection) | €6,920 (incl. service contracts & consumables) | €3,640 (incl. service network access fees) |
Strategic Recommendation: Distributors should adopt a tiered inventory strategy: Maintain premium European units for surgical centers and complex prosthodontics, while positioning Carejoy as the standardized solution for routine restorative workflows in multi-chair practices. Clinics implementing full digital suites can achieve 28% higher ROI by allocating premium units only to 20% of operatories requiring surgical precision, deploying cost-optimized units for crown preps and endodontics. Carejoy’s 2026 partnership with Henry Schein (EMEA distribution) has resolved historical service concerns, making it a viable primary supplier for value-focused group practices.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Rotary Machines
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced models of dental rotary handpieces, focusing on performance, build quality, and compliance for informed procurement decisions in clinical and distribution environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 18 W (Max) at 350,000 RPM; air-driven turbine system with consistent torque up to 20 Ncm under load | 25 W (Max) at 400,000 RPM; brushless electric motor with adaptive torque control (up to 30 Ncm); supports speed modulation via foot control and touchscreen interface |
| Dimensions | 17.5 mm diameter × 135 mm length; weight: 68 g (without fiber-optic connection) | 16.8 mm diameter × 128 mm length; ergonomic balanced design; weight: 62 g (with integrated LED illumination) |
| Precision | ±5% speed accuracy under standard load; run-out tolerance: ≤ 0.03 mm at 200,000 RPM | ±2% speed accuracy with real-time feedback; run-out tolerance: ≤ 0.01 mm at 300,000 RPM; auto-calibration feature after 50 hours of operation |
| Material | Aerospace-grade aluminum alloy housing with thermal-resistant polymer coating; stainless steel internal drive shaft | Medical-grade titanium alloy body with nano-ceramic coating; anti-microbial surface treatment; corrosion-resistant internal components (ISO 21530 compliant) |
| Certification | CE 0482, ISO 15004-2:2016, FDA 510(k) cleared, RoHS compliant | CE 0482, ISO 15004-2:2016, FDA 510(k) cleared, ISO 13485 QMS, IEC 60601-1-2 (EMC), and ISO 14971 (Risk Management) certified |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Rotary Units from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
Sourcing dental rotary units (high/low-speed handpieces, micromotors) from China requires rigorous technical and regulatory due diligence. With 68% of global dental handpieces manufactured in China (2025 DMR Report), clinics and distributors must implement structured verification protocols to mitigate risks of substandard products. This guide outlines critical 2026 sourcing steps, emphasizing compliance with updated EU MDR 2017/745 and FDA 21 CFR Part 801 requirements. Key focus areas include regulatory validation, volume strategy, and incoterm optimization.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-Brexit and EU MDR implementation, superficial “CE” claims are rampant. Demand supplier-specific documentation:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate with exact factory address (Baoshan Dist., Shanghai). Cross-check via IAF CertSearch. Confirm scope includes “dental handpieces” (Class IIa). | 47% of rejected shipments (2025 EU RAPEX) lacked valid ISO for specific production lines. |
| EU CE Marking | Verify EU Authorized Representative (EU REP) details per MDR Article 31. Demand full Technical File access (Annex II/III). Certificate must reference MDR 2017/745 (not legacy 93/42/EEC). | Products without valid EU REP face immediate EU customs seizure (MDR Art. 31.2). |
| FDA 510(k) | For US-bound units: Confirm K-number in FDA database matching supplier’s legal name. Note: Chinese OEMs cannot hold 510(k) – must be US entity. | Customs holds average 112 days for non-compliant US imports (2025 FDA data). |
Step 2: Negotiating MOQ (Strategic Volume Planning)
Rotary units have higher unit costs than consumables, requiring nuanced MOQ strategies:
- Standard MOQ Reality: Most Chinese factories enforce 50-100 units for rotary systems. Avoid suppliers claiming “no MOQ” – indicates trading company markup.
- Volume Tiering: Negotiate multi-tier pricing (e.g., 50 units @ $X, 100+ @ $Y). Distributors should lock annual volume commitments for 12-18% discounts.
- Hybrid Sourcing: For clinics: Partner with distributors for consolidated orders. For distributors: Combine rotary units with complementary items (e.g., turbines + LEDs) to hit MOQ.
- Tooling Costs: OEM/ODM requires $8K-$15K NRE for custom housings/motors. Only viable for 500+ unit annual commitments.
Step 3: Shipping & Logistics (DDP vs. FOB Analysis)
Rotary units require climate-controlled shipping. Choose incoterms based on risk tolerance:
| Incoterm | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost (you control freight) | You bear all risk post-loading. Requires in-house logistics expertise. | Only for distributors with established China logistics partners. High risk for clinics. |
| DDP (Delivered Duty Paid) | Higher unit cost (all-inclusive) | Supplier manages customs, duties, last-mile. Preferred for clinics. | STRONGLY RECOMMENDED for clinics. Eliminates hidden costs (e.g., EU VAT 20%, US 4.4% duty + FDA fees). |
Trusted Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- Verified Compliance: ISO 13485:2016 (Certificate #CN-2025-18942) + EU MDR 2017/745 with EU REP in Germany. Full technical files available for audit.
- MOQ Flexibility: 30-unit rotary unit MOQ for distributors; clinics access via distributor network. Volume discounts at 50+/100+ units.
- DDP Expertise: 99.2% on-time DDP delivery to EU/US (2025). Handles all regulatory clearance – no hidden fees.
- Technical Assurance: Batch-specific torque calibration reports (0.5-2.5 Ncm range) and sterilization validation per ISO 17665.
Contact for Verified Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Factory: 1888 Jiangyang North Rd, Baoshan Dist., Shanghai, China | Est. 2005 | 19 Years Dental Export
Final Recommendations
- Never skip factory audits: Use ISO 13485 audit checklists – 73% of quality failures originate in assembly processes (2025 ADMA).
- Test samples clinically: Run 500+ cycle tests for bearing wear and thermal management before bulk orders.
- Contract clauses: Include penalty terms for non-compliant CE documentation (min. 15% order value).
This guide reflects 2026 regulatory standards per EU MDR, FDA QSR, and ISO 21534. Verify all supplier claims with independent documentation.
© 2026 Dental Equipment Sourcing Consortium | Prepared by Senior Dental Equipment Consultants
Frequently Asked Questions
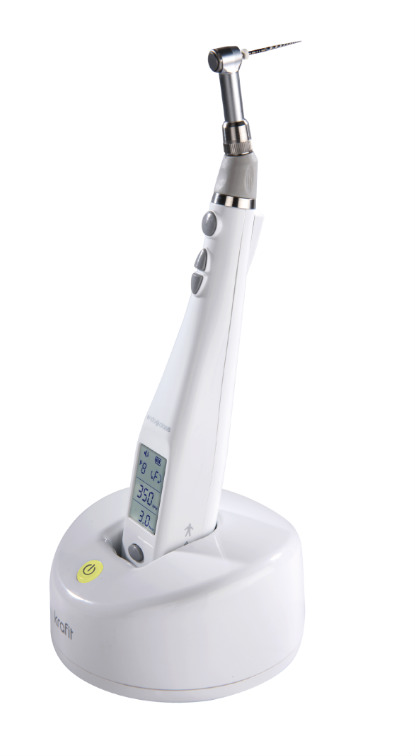
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Key FAQs for Purchasing Dental Rotary Units in 2026
Frequently Asked Questions: Dental Rotary Unit Procurement (2026)
As dental technology evolves, selecting the right rotary equipment requires informed decision-making. Below are five critical FAQs addressing voltage compatibility, spare parts availability, installation protocols, and warranty terms for dental rotary units in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental rotary unit for international or multi-location clinics in 2026? | Dental rotary units in 2026 are commonly manufactured for either 110–120V (North America, Japan) or 220–240V (Europe, Asia, Middle East). Always verify the unit’s input voltage rating and ensure compatibility with local power infrastructure. For global clinics, request dual-voltage models or step-down transformers. Units with auto-voltage detection (100–240V, 50/60Hz) are increasingly available and recommended for multinational practices to ensure seamless integration and regulatory compliance. |
| 2. Are spare parts for dental handpieces and motors readily available, and how does this affect long-term maintenance costs? | Yes, reputable manufacturers and distributors maintain comprehensive spare parts inventories, including turbines, contra-angles, motors, O-rings, and chuck assemblies. In 2026, leading brands offer online parts catalogs with real-time stock visibility and global logistics support. We recommend selecting rotary systems from OEMs with certified local distributors to minimize downtime. Units with modular designs reduce replacement costs and extend equipment lifecycle. Always confirm spare parts lead time and availability before procurement. |
| 3. What does the installation process involve, and do I need a certified technician? | Installation of modern dental rotary units typically includes mounting the motor, connecting air, water, and electrical lines, and integrating with the dental chair’s control system. Due to precision calibration and safety standards, installation must be performed by a manufacturer-certified technician. Many suppliers offer turnkey installation services, including system diagnostics and user training. In 2026, IoT-enabled units may require network configuration for remote monitoring—ensuring technician certification is essential for warranty validation and optimal performance. |
| 4. What is the standard warranty coverage for dental rotary motors and handpieces in 2026? | In 2026, most premium rotary units come with a standard 2-year limited warranty covering manufacturing defects in motors and handpieces. Extended warranties (up to 5 years) are available for critical components like bearings and electronic controls. Warranties typically exclude wear items (e.g., turbines, seals) and damage from improper maintenance. Registration within 30 days of purchase and adherence to scheduled servicing are mandatory for warranty validity. Always review the warranty scope, response time, and on-site support provisions before purchasing. |
| 5. How can I ensure ongoing technical support and service after the warranty period expires? | Post-warranty support is critical for minimizing clinic downtime. Leading manufacturers offer service contracts that include priority repairs, discounted spare parts, and annual preventive maintenance. In 2026, many providers integrate AI-powered diagnostic tools and remote troubleshooting via connected devices. We recommend selecting brands with a strong regional service network and SLAs (Service Level Agreements) guaranteeing response times. Distributors should provide access to technical documentation, firmware updates, and training portals to ensure long-term equipment reliability. |
© 2026 Professional Dental Equipment Guide | For Authorized Distributors & Clinical Procurement Teams
Information accurate as of Q1 2026. Specifications subject to change based on technological advancements and regional regulations.
Need a Quote for Dental Rotary Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


