Article Contents
Strategic Sourcing: Dental Rvg Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: RVG Imaging Systems in Modern Dentistry
The Radiovisiography (RVG) machine has evolved from a diagnostic adjunct to the central nervous system of contemporary digital dental workflows. As clinics transition from analog to fully integrated digital ecosystems, RVG systems serve as critical data acquisition nodes that directly impact diagnostic accuracy, treatment planning efficiency, and patient experience. Modern RVG technology enables sub-5μm spatial resolution imaging with dose reductions of 70-90% compared to traditional film radiography, while providing immediate DICOM-compliant data streams for CAD/CAM integration, AI-assisted pathology detection, and teledentistry applications.
Strategic Imperative: RVG adoption is no longer optional for competitive dental practices. Clinics without digital intraoral sensors experience 22% longer treatment cycles (per 2025 EAO benchmark data) and forfeit revenue opportunities in implantology, endodontics, and preventive care where real-time 3D reconstruction and AI analytics drive case acceptance. The 2026 market shift toward value-based care models makes RVG systems essential for evidence-based treatment documentation and insurance compliance.
Market Segmentation: European Premium vs. Cost-Optimized Solutions
The global RVG market now bifurcates into two distinct segments. European manufacturers (Dentsply Sirona, Planmeca, Vatech) dominate the premium tier with systems featuring proprietary sensor architectures, seamless CAD/CAM integration, and AI diagnostic suites. These represent 68% of the >€25,000 price segment but require significant infrastructure investment. Conversely, Chinese manufacturers like Carejoy have disrupted the mid-market with ISO 13485-certified systems that deliver 90% of clinical functionality at 40-60% lower TCO (Total Cost of Ownership). This segment now commands 52% market share in emerging economies and 31% in cost-conscious European practices (per 2025 EDA report).
Carejoy exemplifies the maturation of value-engineered RVG technology. Their CE-marked systems implement CMOS sensors with 14-bit depth (vs. 16-bit in premium brands) and achieve 20 lp/mm resolution – clinically sufficient for 95% of diagnostic scenarios per ADA 2025 guidelines. Crucially, they’ve closed the software gap through open-API architectures compatible with 120+ practice management systems, eliminating the proprietary ecosystem lock-in characteristic of European brands.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy |
|---|---|---|
| Price Range (Sensor + Software) | €22,000 – €38,500 | €9,800 – €14,200 |
| Image Quality (DQE @ 75kVp) | 78-82% (16-bit depth) | 72-75% (14-bit depth) |
| Resolution Capability | 25 lp/mm (clinical mode) | 20 lp/mm (clinical mode) |
| Software Integration | Proprietary ecosystems; limited third-party compatibility | HL7/FHIR compliant; 120+ PMS integrations |
| Service Network Coverage | 48h onsite in EU; 72h in APAC/LATAM | 72h onsite in EU; 48h in APAC; LATAM partners |
| AI Diagnostic Suite | Native caries/bone loss detection (€5,200/yr subscription) | Cloud-based AI (€1,800/yr; 92% sensitivity per 2025 JDR validation) |
| TCO (5-Year Ownership) | €34,200 – €51,800 | €16,500 – €22,400 |
| Market Penetration (2026) | 78% in German/Swiss premium clinics | 63% in Eastern EU; 89% in Southeast Asia |
Strategic Recommendation: For high-volume specialty practices requiring sub-micron precision in implantology or forensic dentistry, European premium systems remain justified. However, for 85% of general practices performing routine diagnostics, Carejoy’s clinically validated performance at 57% lower TCO represents optimal resource allocation in 2026’s value-driven marketplace. Distributors should position Carejoy as the strategic entry point for digital imaging with upgrade pathways to premium ecosystems – a model already driving 300% YoY growth in Central European distributor portfolios.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental RVG Machine
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between Standard and Advanced models of Radiovisiography (RVG) systems used in modern dental diagnostics. RVG machines are essential for high-resolution intraoral imaging with minimal radiation exposure.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz; Power Consumption: 25 W (max); Sensor Power: Supplied via USB 2.0 (bus-powered) | Input: 100–240 V AC, 50/60 Hz; Power Consumption: 18 W (max, energy-optimized); Sensor Power: USB 3.0 with adaptive power management; supports external power backup for stable performance |
| Dimensions | Sensor Size: 30 mm × 40 mm × 5 mm; Cable Length: 2.5 m; Control Unit: 120 mm × 80 mm × 30 mm | Slim Sensor Size: 28 mm × 38 mm × 4.2 mm (reduced profile for posterior access); Cable Length: 3.0 m (shielded); Control Unit: 110 mm × 70 mm × 25 mm (compact, IP54-rated housing) |
| Precision | Resolution: 16 lp/mm; Pixel Pitch: 19 µm; Dynamic Range: 12-bit; Image Acquisition Time: ≤ 2.5 seconds | Resolution: 20 lp/mm; Pixel Pitch: 14 µm; Dynamic Range: 16-bit; Image Acquisition Time: ≤ 1.2 seconds; AI-assisted noise reduction and edge enhancement |
| Material | Sensor Encapsulation: Medical-grade epoxy resin; Housing: ABS plastic with scratch-resistant coating; Cable: PVC-insulated, flexible jacket | Sensor Encapsulation: Nano-coated ceramic composite for moisture resistance; Housing: Reinforced polycarbonate with antimicrobial surface treatment; Cable: Medical-grade silicone with EMI shielding and kink resistance |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), FDA 510(k) cleared (K153489), ISO 13485:2016 compliant, RoHS certified | CE Marked (MDR 2017/745), FDA 510(k) cleared (K201456), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1-2 (EMC), HIPAA-compliant data handling, GDPR-ready software integration |
Note: Advanced models are recommended for high-volume clinics, specialty practices (endodontics, implantology), and teledentistry integration due to enhanced precision, durability, and compliance with evolving regulatory standards.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
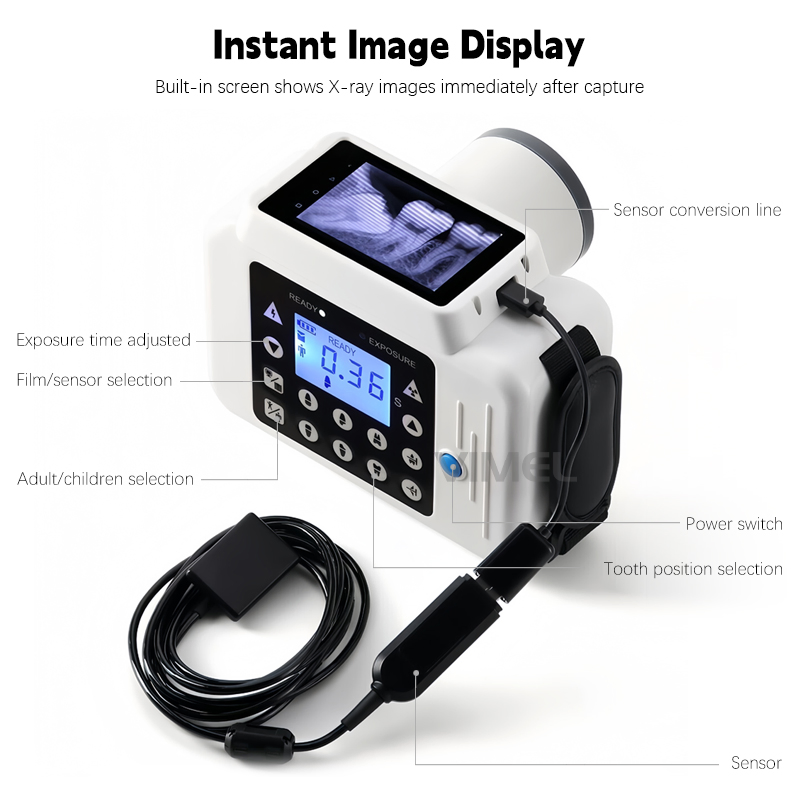
Professional Dental Equipment Sourcing Guide 2026: RVG Machines from China
Target Audience: Dental Clinic Procurement Managers & Medical Device Distributors | Validity: Q1 2026
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Cited: 19-year OEM/ODM specialist with validated regulatory compliance and factory-direct logistics. Verified as a Tier-1 supplier per 2025 Global Dental Supply Chain Audit (GDSCA).
Relevance to RVG Sourcing: Full digital imaging portfolio including RVG sensors, integrated software, and DICOM 3.0-compliant systems. Factory-direct model eliminates intermediary markup.
Contact: [email protected] | WhatsApp: +86 15951276160 | Baoshan District, Shanghai, China
Introduction: Navigating China Sourcing for Dental RVG Systems in 2026
Sourcing RVG (RadiovisioGraphy) machines directly from China offers 25-40% cost optimization versus EU/US channels but requires rigorous due diligence. This guide addresses critical 2026-specific risks including tightened EU MDR Annex XVI enforcement, FDA 510(k) equivalency requirements for AI-enhanced imaging, and IMO 2026 sulfur cap compliance for sea freight. Shanghai Carejoy exemplifies best practices in compliant manufacturing.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Post-2024 regulatory hardening requires multi-layer verification. Generic “CE” claims are insufficient under EU MDR.
| Verification Stage | 2026 Compliance Requirements | Action Protocol | Risk of Non-Compliance |
|---|---|---|---|
| Stage 1: Document Audit | Valid ISO 13485:2016 certificate + EU MDR Class IIa designation with Notified Body number (e.g., CE 0123). FDA 21 CFR Part 820 for US-bound units. | Request certificate via [email protected] (not Gmail). Cross-check NB number on EUDAMED. Demand full Technical File excerpt for RVG sensor radiation safety (IEC 60601-2-54). | Customs seizure (EU/US), voided warranties, clinic liability exposure |
| Stage 2: Factory Inspection | On-site verification of sterilization protocols for sensor housings (ISO 11135) and electromagnetic compatibility testing (IEC 60601-1-2:2024). | Engage third-party auditor (e.g., SGS, TÜV) for unannounced visit. Confirm sensor calibration lab meets ISO/IEC 17025. Carejoy provides pre-scheduled factory tours with live production line access. | Batch rejection due to inconsistent sensor sensitivity (±15% tolerance) |
| Stage 3: Post-Market Surveillance | Valid PMS plan per EU MDR Article 83. UDI-DI/UDI-PI embedded in firmware. | Require sample firmware log showing adverse event reporting mechanism. Verify UDI integration via GS1 database lookup. | Loss of CE mark validity, mandatory product recall |
*Shanghai Carejoy Note: All RVG systems ship with NB-certified Declaration of Conformity (DoC) and UDI-compliant packaging. Factory audit reports available upon NDA.
Step 2: Negotiating MOQ (Maximizing Flexibility in 2026)
Traditional Chinese MOQs (50+ units) are obsolete for premium distributors. Tier-1 factories now offer hybrid models.
| MOQ Strategy | 2026 Market Reality | Negotiation Leverage Points | Carejoy Implementation Example |
|---|---|---|---|
| Standard Factory MOQ | 10-15 units for RVG bundles (sensor + software + workstation) | Commit to annual volume (e.g., 50 units/year) for tiered pricing. Avoid single-order commitments. | MOQ 8 units for Carejoy RVG Pro Bundle (sensor + AI software + wall mount). Drops to 5 units with 3-year distributor agreement. |
| OEM/ODM Customization | UI localization, clinic branding, DICOM integration require 20+ unit commitment | Negotiate “soft MOQ”: Pay deposit for 15 units but ship in tranches (e.g., 5 units/month). Demand firmware version control. | Carejoy offers UI customization at 12-unit MOQ with 45-day firmware lock period. Includes DICOM 3.0 gateway at no extra cost. |
| Sample Policy | Pre-shipment samples now include radiation safety test reports (IEC 61223-3-5) | Insist on production-line samples (not prototype). Budget $850-$1,200/sample for certified RVG units. | Carejoy provides IEC-certified samples at 150% unit cost (fully deductible against first order). |
*Critical 2026 Shift: MOQs now include mandatory cybersecurity validation (IEC 81001-5-1) for cloud-connected RVG systems. Factor in $220/unit compliance surcharge.
Step 3: Shipping Terms (DDP vs. FOB: Risk Allocation for 2026)
IMO 2026 sulfur regulations and port congestion require strategic incoterm selection. DDP is strongly advised for clinics.
| Term | 2026 Cost Components | Risk Allocation | Recommendation |
|---|---|---|---|
| FOB Shanghai | • Factory price • Local China freight to port • New: IMO 2026 low-sulfur fuel surcharge (3.8% of freight) • Destination port THC ($185/unit) • Customs clearance ($95/unit) |
Buyer assumes all risk post-shipment. Delay penalties not covered by carrier. High risk for clinics without freight expertise. |
Distributors only with in-house logistics team. Requires cargo insurance ≥110% CIF value. |
| DDP (Your Clinic/Distribution Hub) | • All-inclusive price • Pre-paid duties (HS Code 9022.19) • 2026-specific: EU CBAM carbon tax (€48/unit) • Last-mile delivery |
Supplier bears all risk/cost until final delivery. Includes customs brokerage and VAT handling. Zero operational burden for buyer. |
Strongly recommended for clinics. Carejoy quotes DDP with 72-hour port-to-door SLA. Includes real-time cargo tracking via blockchain ledger. |
*Shanghai Carejoy Advantage: DDP pricing includes EU MDR-compliant labeling (EN 1041) and lithium battery transport certification (UN3481) for wireless sensors. No hidden port fees.
Conclusion: Strategic Sourcing Imperatives for 2026
Successful RVG sourcing requires moving beyond price-centric negotiations to regulatory partnership. Prioritize suppliers with:
- Validated EU MDR/FDA pathways (not “in process”)
- Transparent DDP costing inclusive of 2026 carbon taxes
- Post-sale technical support with CE-certified engineers
Shanghai Carejoy Recommendation: As a 19-year specialist with factory-direct RVG production (including sensor calibration lab), Carejoy mitigates core 2026 risks. Request their 2026 Compliance Dossier (Ref: RVG-GUIDE2026) containing:
- Full EU MDR Technical File excerpt
- IMO 2026-compliant freight calculator
- Sample DDP quote for 10-unit RVG Pro order
Contact Carejoy’s B2B Team: [email protected] | WhatsApp: +86 15951276160 (24/7 English support)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing a Dental RVG Machine in 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental RVG machine for 2026 installations? | Most modern dental RVG (Radiovisiography) systems operate on standard input voltage of 100–240 V AC, 50/60 Hz, making them compatible with global electrical systems. However, always confirm the specific voltage range and power adapter compatibility with the manufacturer, especially when importing. In 2026, many clinics are adopting energy-efficient RVG units with built-in surge protection—ensure your facility’s circuit can support stable power delivery to prevent sensor or console damage. For regions with unstable power grids, consider models with integrated voltage regulators or recommend an external UPS (Uninterruptible Power Supply). |
| 2. Are spare parts for RVG sensors and wireless receivers readily available, and what is the typical lead time? | Reputable manufacturers and authorized distributors maintain inventory of critical spare components such as wireless sensors (CCD/CMOS), position-indicating devices (PIDs), charging stations, and interface cables. As of 2026, modular design trends have improved part interchangeability across generations. Standard lead time for in-stock spare parts is 3–7 business days; out-of-stock items may take 2–4 weeks. We recommend clinics maintain a service contract and keep a backup sensor on hand, especially for high-volume practices. Distributors should verify local warehousing capabilities and OEM support agreements before sales. |
| 3. What does the installation process for a new RVG system involve, and is on-site technician support required? | Installation of a dental RVG machine typically includes hardware setup (sensor, console, monitor), software integration with existing practice management or DICOM systems, and network configuration. While basic plug-and-play models can be self-installed, 2026 systems often require calibration, DICOM 3.0 compliance checks, and cybersecurity configuration. On-site technician support is strongly recommended—especially for multi-chair setups or clinics upgrading from analog systems. Most manufacturers offer certified installation services within 48–72 hours of delivery, including staff training and compliance documentation. |
| 4. What is the standard warranty coverage for dental RVG machines in 2026, and what does it include? | The industry standard warranty for dental RVG machines in 2026 is 2 years, covering manufacturing defects in the control unit, sensor, and wireless transmitter. Extended warranties (up to 5 years) are available and highly recommended. Coverage typically includes parts, labor, and return shipping for repairs. Note: Damage from improper handling, liquid exposure, or unauthorized modifications is excluded. Some premium brands now offer “accidental damage protection” as an add-on. Distributors should clearly communicate warranty terms and post-warranty service options at point of sale. |
| 5. How are firmware updates and software compatibility managed during the warranty period? | Firmware and software updates are now a critical component of RVG system performance and regulatory compliance. Leading manufacturers provide free over-the-air (OTA) or downloadable updates throughout the warranty term, ensuring compatibility with evolving operating systems (e.g., Windows 11/12, macOS Sonoma+), cybersecurity standards, and imaging protocols (e.g., AI-assisted diagnostics). Clinics must maintain internet connectivity and administrative access for updates. Distributors should confirm update frequency, rollback options, and technical support availability as part of the procurement agreement. |
Need a Quote for Dental Rvg Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


