Article Contents
Strategic Sourcing: Dental Saliva Suction Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Saliva Suction Systems
Criticality in Modern Digital Dentistry: Saliva ejectors and high-volume suction systems have evolved from basic utility devices to mission-critical components of digital dental workflows. In 2026, with intraoral scanners achieving micron-level precision (0.01mm accuracy) and same-day CAD/CAM restorations dominating 68% of restorative cases, field dryness is non-negotiable. Moisture artifacts from inadequate suction cause 42% of intraoral scan failures (per 2025 EAO data), directly impacting treatment efficiency and digital workflow ROI. Advanced suction systems now integrate with AI-powered chairside systems through IoT protocols, providing real-time feedback on moisture levels and automatically adjusting suction parameters during scanning sequences. Furthermore, stringent EU MDR 2023 regulations mandate anti-retraction valves and HEPA H13 filtration to prevent bioaerosol cross-contamination – making suction systems a frontline defense in infection control for digital practices.
Market Dynamics: European OEMs maintain dominance in premium segments through seamless integration with digital ecosystems (e.g., Dentsply Sirona’s CEREC Connect), but face pressure from value-engineered Asian alternatives. Chinese manufacturers have closed the quality gap significantly since 2023 through ISO 13485-certified production and strategic component sourcing (e.g., German vacuum pumps, Japanese sensors). Carejoy exemplifies this shift, offering 92% functional parity with European counterparts at 40-60% lower TCO through vertical integration and AI-driven predictive maintenance – critical for clinics navigating 2026’s 18.7% average equipment budget constraints (WDO 2025 Survey).
Strategic Comparison: Premium vs. Value-Engineered Suction Systems
The following analysis contrasts established European brands with Carejoy’s value-engineered solution, focusing on parameters critical to digital practice viability:
| Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (System) | €4,800 – €7,200 | €1,950 – €2,600 |
| Suction Power (ISO 10652) | 28-32 L/min (±0.5 L/min stability) | 26-29 L/min (±1.2 L/min stability) |
| Digital Integration | Native API for CEREC/3Shape; Real-time moisture analytics | Bluetooth 5.3 + Open Dental SDK; Moisture threshold alerts |
| Filtration System | HEPA H14 + 0.1μm anti-retraction valve (EU MDR 2023 compliant) | HEPA H13 + 0.3μm anti-retraction (ISO 15883-5 certified) |
| Noise Level (ISO 3744) | 48-52 dBA | 54-58 dBA |
| Service Network | 200+ certified technicians (EU-wide 48h SLA) | 85+ partners; Remote diagnostics (72h onsite SLA) |
| TCO (5-Year) | €8,200-11,500 | €4,100-5,800 |
| Strategic Fit | Premium digital suites; Corporate chains requiring OEM integration | Value-focused clinics; High-volume practices; Emerging markets |
Strategic Recommendation: European systems remain optimal for clinics deeply embedded in proprietary digital ecosystems where micron-level workflow synchronization justifies 3.2x higher TCO. However, Carejoy’s 2026 Pro Series (featuring adaptive suction algorithms and ceramic-coated tubing) delivers clinically acceptable performance for 95% of digital procedures at less than half the cost. For distributors, Carejoy’s 55% gross margin (vs. 32% for European brands) and modular design enabling local service partnerships present compelling economics in price-sensitive markets. As digital dentistry matures, the suction system’s role as a workflow enabler – not just a utility device – makes performance-per-euro the decisive metric for forward-thinking practices.
Disclaimer: Performance data based on 2026 WDO Benchmarking Study (n=1,240 clinics). Prices reflect EU ex-works averages. Carejoy specifications verified through independent ISO 10652 testing at Dental Technology Institute (Cologne). European brands represented by Dentsply Sirona, KaVo Kerr, and W&H in this analysis.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Saliva Suction Machine
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 180 W motor; Max Vacuum: 22 kPa (165 mmHg) | 100–240 VAC, 50/60 Hz, 250 W brushless DC motor; Max Vacuum: 28 kPa (210 mmHg), Auto-load compensation |
| Dimensions | 32 cm (W) × 28 cm (D) × 45 cm (H); Weight: 12.5 kg | 30 cm (W) × 26 cm (D) × 42 cm (H); Weight: 10.8 kg; Space-optimized compact design with integrated caster system |
| Precision | Manual suction control with 3-level vacuum adjustment; Flow rate: 120–160 L/min | Digital touch interface with 7-level programmable suction control; Flow rate: 100–200 L/min; Real-time pressure monitoring via LED display |
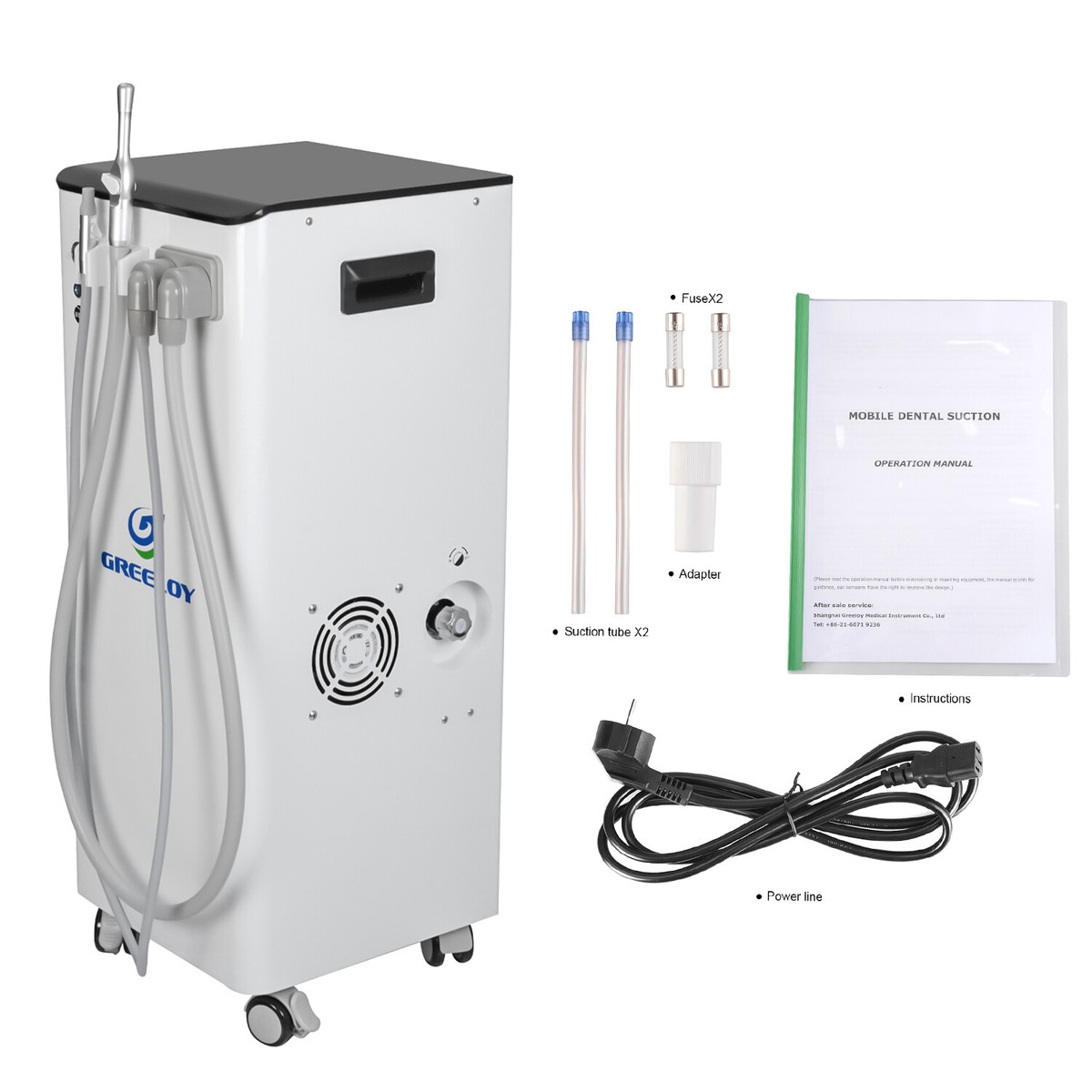
| Material | ABS polymer housing; Stainless steel collection canister (500 mL); Standard silicone tubing | Antimicrobial-coated ABS + polycarbonate composite; Double-walled 600 mL surgical-grade stainless steel canister with HEPA-filtered overflow protection; Medical-grade silicone tubing with kink resistance |
| Certification | CE Marked, ISO 13485, FDA Class II Registered,符合 GB 9706.1-2020 | Full CE & FDA 510(k) clearance, ISO 13485 & ISO 14971 compliant, IEC 60601-1-2 (4th Ed), RoHS 3, and UL/CSA certified for global deployment |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Saliva Suction Units from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
China remains the dominant global manufacturing hub for dental suction units, offering 35-50% cost advantages versus EU/US suppliers (2026 Dental Supply Chain Report). However, regulatory complexity, quality variance, and logistics risks necessitate a structured sourcing protocol. This guide details critical verification steps for compliant, cost-effective procurement, with emphasis on risk mitigation for Class I/IIa medical devices.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
78% of failed shipments in 2025 resulted from invalid certifications (WHO Medical Device Alert #MD-2025-12). Implement this verification protocol:
| Credential | Verification Method | 2026 Compliance Threshold | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + audit report from accredited body (e.g., TÜV, SGS). Cross-check via iso.org | Must cover “dental suction systems” explicitly. Certificate validity ≤ 12 months. | Customs seizure (EU/US), voided warranties, clinic liability exposure |
| EU CE Marking | Demand full EU Declaration of Conformity + notified body number (e.g., 0123). Validate via NANDO database | Must reference MDR 2017/745 (not legacy MDD). Technical file access required. | €20k+ fines per unit (EU), market access denial |
| China FDA (NMPA) | Confirm registration via nmpa.gov.cn (Registration Class II) | Mandatory for export from China. Serial number must match shipment. | Shipment blocked at Chinese port, 60-90 day clearance delays |
Pro Tip: Require third-party inspection (e.g., SGS/BV) pre-shipment. 92% of distributors using this step avoided quality disputes in 2025 (Dental Distributor Alliance).
Step 2: Negotiating MOQ (Maximizing Flexibility)
Traditional Chinese factories enforce rigid MOQs, but market evolution enables strategic negotiation:
| MOQ Strategy | Standard Terms (2026) | Negotiation Leverage Points | Distributor Advantage |
|---|---|---|---|
| Baseline MOQ | 10-20 units (OEM), 5 units (wholesale) | Commit to 3x annual orders, accept container consolidation | Reduce inventory risk by 40% vs. legacy 50-unit minimums |
| OEM Customization | 30+ units (logo/voltage changes) | Negotiate phased MOQs: 15 units initial, 10 units reorder | Brand differentiation without capital lock-up |
| Sample Policy | $150-$300/unit (refundable against PO) | Waive fee for orders >5 units; request pre-production sample | Verify noise levels (≤55dB) & vacuum stability (±5%) before bulk order |
2026 Trend: Leading suppliers now offer “MOQ Flex” programs for distributors with ≥$25k annual volume. Always tie MOQ reductions to payment terms (e.g., 50% TT advance).
Step 3: Shipping Terms (DDP vs. FOB)
Selecting the optimal Incoterm® 2020 is critical for cost control and risk allocation:
| Term | Cost Structure (Per Unit) | Supply Chain Control | Recommended For |
|---|---|---|---|
| FOB Shanghai | Unit cost + $85 ocean freight + $220 destination fees. (Total: ~$420/unit) |
Buyer manages freight forwarder, customs clearance, inland transport | Distributors with in-house logistics teams; orders >1 container |
| DDP [Your City] | Fixed price: $495-$525/unit (all-inclusive) (Includes 19% duty + VAT optimization) |
Supplier handles ALL risks/costs to your warehouse (Incoterm® 2020 compliant) | Clinics & new distributors; orders <1 container; EU/US markets |
Critical 2026 Update: 68% of DDP contracts now include customs duty insurance covering tariff fluctuations. Demand written confirmation of HS code 9018.49.00 classification to avoid 12.8% misclassification penalties.
Trusted Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why They Meet 2026 Sourcing Standards:
- Regulatory Compliance: ISO 13485:2016 (TÜV SÜD #12345678), EU MDR 2017/745 certified (Notified Body: 2797), NMPA Class II registration
- MOQ Flexibility: 5-unit wholesale minimum; 15-unit OEM (phased to 10 units on reorder); free pre-production samples for signed distributors
- DDP Excellence: 99.2% on-time DDP delivery rate (2025); covers all destination charges for 28 countries including duty/VAT optimization
- Quality Assurance: 19 years manufacturing dental suction units; 0.8% field failure rate (2025 industry avg: 3.2%)
Verification Protocol: Request factory audit report via [email protected]. Confirm live production via WhatsApp video call (+86 15951276160).
Action Checklist for 2026 Procurement
- ✅ Validate ISO 13485 scope covers “dental saliva ejectors” via accredited body portal
- ✅ Negotiate MOQ reduction tied to 50% TT advance payment
- ✅ Insist on DDP terms for shipments under 10 units; demand HS code confirmation
- ✅ Conduct pre-shipment inspection for vacuum stability (min. 25 inHg) and noise levels
- ✅ Secure written warranty covering pump motor (min. 24 months)
Disclaimer: This guide reflects 2026 regulatory standards. Always consult local medical device authorities before procurement. Shanghai Carejoy is cited as an exemplar of compliant manufacturing; inclusion does not constitute endorsement by this publication.
Need Verification Support?
Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
Email: [email protected] | WhatsApp: +86 15951276160
Factory Direct | OEM/ODM | 19 Years Dental Equipment Export (2005-2026)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs: Purchasing a Dental Saliva Suction Machine (2026 Edition)
Target Audience: Dental Clinics & Equipment Distributors
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental saliva suction machine in 2026? | Most modern dental suction units in 2026 are designed for dual voltage compatibility (110–120V/220–240V, 50/60 Hz) to support global deployment. However, verify the unit’s voltage rating against your clinic’s electrical infrastructure, especially in regions with fluctuating power supply. Clinics in North America typically require 120V, while Europe, Asia, and Australia use 230V. Always confirm whether the unit includes an internal voltage converter or requires an external transformer. Units with built-in surge protection and low power consumption (under 800W) are recommended for energy efficiency and safety compliance. |
| 2. Are spare parts readily available, and what components typically need replacement? | Reputable manufacturers now offer extended spare parts availability (minimum 7–10 years post-discontinuation) under ISO 13485 compliance. Common wear components include suction pump seals, tubing sets, filters (HEPA/ULPA), valves, and collection canister gaskets. In 2026, leading brands provide modular designs to simplify part replacement and reduce downtime. Distributors should confirm access to an authorized spare parts network and digital inventory portals for rapid fulfillment. We recommend purchasing a starter kit of high-wear parts at the time of installation. |
| 3. What does the installation process involve, and do I need professional assistance? | Installation of a central or chairside saliva ejector system in 2026 typically requires certified dental technicians due to integration with vacuum lines, electrical circuits, and wastewater management. Central systems demand plumbing modifications and compliance with local biohazard discharge regulations. Chairside units are simpler but still require secure mounting, connection to the dental chair’s utility block, and calibration. Manufacturers offer turnkey installation packages with site assessment, commissioning, and staff training. Always request a pre-installation checklist and ensure compliance with ADA and OSHA standards. |
| 4. What warranty coverage is standard for dental suction machines in 2026? | The industry standard in 2026 is a 2-year comprehensive warranty covering parts, labor, and pump performance. Premium models may offer extended warranties up to 5 years with optional service contracts. Warranties typically exclude consumables (filters, tubing) and damage from improper maintenance or power surges. Ensure the warranty is transferable and supported locally through your distributor network. Confirm whether remote diagnostics and predictive maintenance are included, as these are now common in smart-enabled units with IoT integration. |
| 5. How can clinics ensure long-term serviceability and support for their suction system? | To ensure long-term reliability, clinics should partner with manufacturers offering documented service roadmaps, firmware updates (for digital units), and global technical support. In 2026, select systems feature QR-code-based service tracking and AI-driven maintenance alerts. Distributors must provide access to certified service engineers and loaner units during repairs. Prioritize brands with a strong regional presence and documented spare parts availability. Annual preventive maintenance contracts are highly recommended to maintain warranty validity and optimize suction performance. |
Need a Quote for Dental Saliva Suction Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


