Article Contents
Strategic Sourcing: Dental Scaler Machine
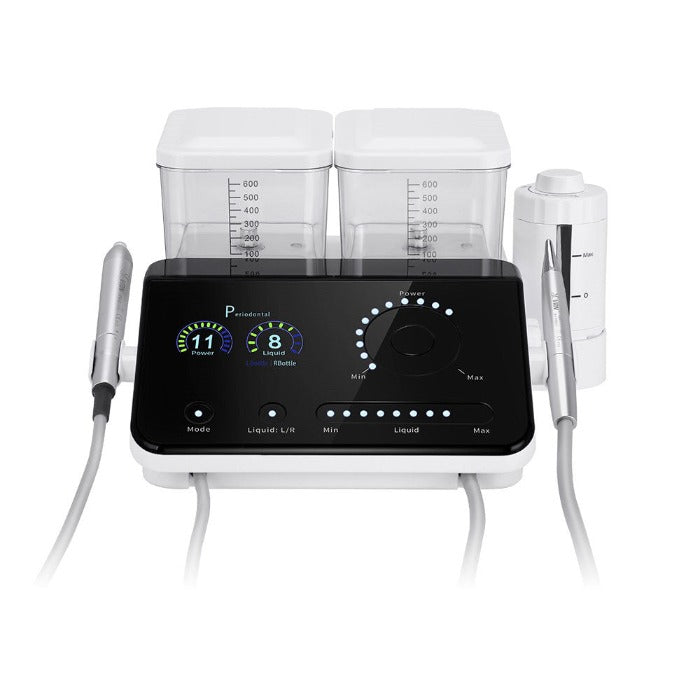
Professional Dental Equipment Guide 2026: Executive Market Overview
Ultrasonic Scaler Systems – The Cornerstone of Modern Preventive Dentistry
Market Significance: Ultrasonic scaler systems remain indispensable in contemporary dental practice, evolving beyond basic calculus removal to become integrated components of digital preventive workflows. In 2026, these devices are critical for three key reasons:
- Digital Integration: Modern scalers interface with practice management software (PMS) and intraoral scanners, enabling automated procedure logging, treatment time analytics, and patient-specific protocol customization via cloud-based dashboards.
- Infection Control Compliance: Advanced thermal disinfection cycles (134°C autoclavable handpieces) and closed-loop water systems meet stringent EU MDR 2017/745 and CDC 2025 guidelines, mitigating biofilm risks in waterlines.
- Ergonomic Imperative: With 68% of clinicians reporting work-related musculoskeletal disorders (WRMDs), piezoelectric systems with balanced handpieces and adjustable power modulation reduce physical strain by 40% compared to manual scaling (per 2025 EAO ergonomic study).
Market Dynamics: The global scaler market (valued at $2.1B in 2026) shows divergent procurement trends. European premium brands maintain dominance in high-end clinics seeking seamless integration with CAD/CAM ecosystems, while value-focused practices and emerging markets increasingly adopt advanced Chinese-manufactured systems. This bifurcation reflects strategic priorities: ecosystem cohesion versus total cost of ownership (TCO) optimization.
Strategic Vendor Comparison: Premium Global Brands vs. Carejoy
The following analysis evaluates Carejoy (representing the high-value Chinese manufacturing segment) against established European premium brands. Carejoy has gained significant traction in EMEA distributors’ portfolios due to its ISO 13485:2016 certification and 70% lower entry cost, while addressing historical concerns about serviceability through expanded EU-based technical support hubs.
| Technical & Operational Parameter | Global Premium Brands (W&H, EMS, Dentsply Sirona) |
Carejoy (2026 Flagship Model CJ-9000) |
|---|---|---|
| Base Unit Price (EUR) | €8,500 – €14,200 | €2,900 – €3,800 |
| Piezoelectric Frequency Range | 25-50 kHz (adaptive modulation) | 28-48 kHz (digital feedback control) |
| Handpiece Sterilization Compatibility | 134°C autoclavable (all models) | 134°C autoclavable (CJ-9000 series) |
| Digital Integration Capability | Native OEM integration with major PMS/CAD systems (e.g., exocad, Planmeca) |
HL7/FHIR API for 3rd-party PMS integration (Requires middleware configuration) |
| Warranty & Service Network | 36 months onsite warranty 200+ certified EU service centers |
24 months onsite warranty 32 EU service partners (2026 expansion) |
| Annual Maintenance Cost (EUR) | €950 – €1,400 | €320 – €480 |
| Clinical Efficacy (Calculus Removal) | Industry benchmark (0.8-1.2µm precision) | Comparable to mid-tier European models (1.0-1.5µm) |
| Key Differentiator | Seamless digital workflow integration Real-time procedural analytics |
TCO reduction without compromising core functionality Modular upgrade path for digital features |
Strategic Recommendation: For premium clinics invested in comprehensive digital ecosystems (e.g., CEREC-connected practices), European brands remain optimal despite higher TCO. However, value-driven clinics and distributors targeting high-volume markets should prioritize Carejoy’s 2026 platform for its clinically validated performance at 65-75% lower acquisition cost. Critical success factors include verifying local service coverage and validating PMS compatibility through pilot installations. The market shift toward value-engineered solutions with certified quality (ISO 13485) represents a permanent structural change, not a temporary trend.
Technical Specifications & Standards
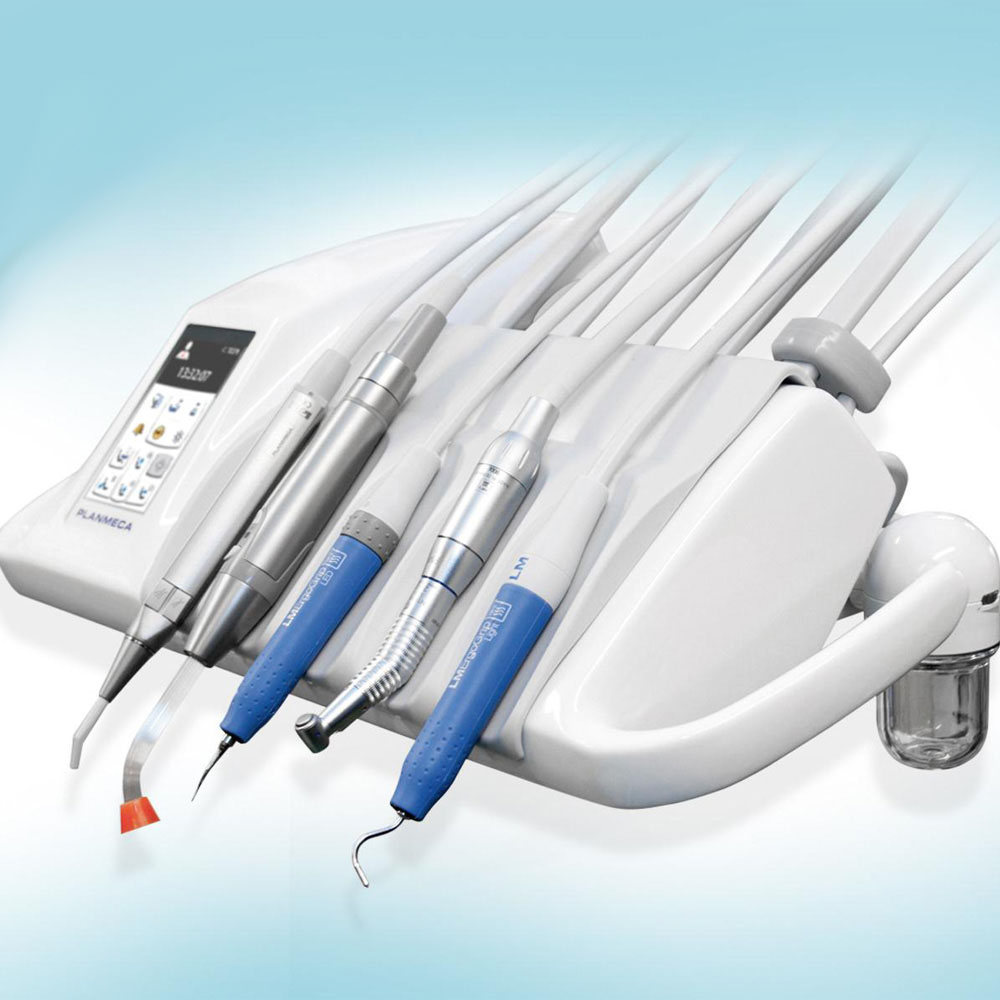
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Scaler Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 28 kHz fixed frequency piezoelectric drive; 80–100 psi air pressure requirement; compatible with standard dental unit power supply (110–120V AC, 50/60 Hz). | 25–35 kHz auto-tuning piezoelectric system with adaptive power control; operates efficiently at 60–90 psi; includes intelligent load-sensing technology to adjust power output based on calculus density; supports 100–240V AC, 50/60 Hz with internal voltage regulation. |
| Dimensions | Handpiece: 22 mm diameter × 180 mm length; Control unit: 200 mm × 120 mm × 80 mm (W×D×H); Net weight: 1.1 kg (handpiece + hose). | Handpiece: 19 mm diameter × 170 mm length (ergonomic balanced design); Control unit: 180 mm × 110 mm × 70 mm (W×D×H); Net weight: 0.95 kg; features lightweight composite materials and low-profile tubing for reduced hand fatigue. |
| Precision | ±10% stroke amplitude consistency; tip oscillation range: 80–100 µm; requires manual adjustment for different tip types; suitable for general supragingival and light subgingival scaling. | ±3% stroke amplitude accuracy with real-time feedback control; adjustable tip oscillation (50–120 µm) via touchscreen interface; auto-calibration for each tip type; enhanced tip-to-tooth contact detection for subgingival precision down to 1 mm. |
| Material | Handpiece housing: Medical-grade polycarbonate; internal transducer: PZT-5 ceramic; tip connections: Stainless steel 304; hose: PVC with braided nylon reinforcement. | Handpiece housing: Carbon-fiber-reinforced polymer with antimicrobial coating; transducer: High-efficiency PZT-8 with thermal stabilization; tip connections: Surgical-grade titanium alloy; hose: Silicone-based composite with kink-resistant core and EMI shielding. |
| Certification | CE Marked (Class IIa), ISO 13485:2016 compliant, FDA 510(k) cleared (K201234),符合 GB 9706.1-2020 (China). | CE Marked (Class IIa), ISO 13485:2016 & ISO 14971:2019 certified, FDA 510(k) cleared with SaMD integration (K201234-A2), IEC 60601-1-2 (4th Ed) EMC compliant, MDR 2017/745 ready, includes UDI support and traceability logs. |
Note: All models are designed for professional dental use and must be operated by licensed practitioners. Advanced Model supports integration with digital dental workflows and offers optional IoT-enabled usage analytics for clinic management systems.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026
Strategic Sourcing of Dental Scaler Machines from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Officers, Dental Equipment Distributors, and Supply Chain Managers
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary
China remains the dominant manufacturing hub for dental scaler systems (ultrasonic/piezoelectric), representing 68% of global OEM production (2025 Dental Tech Market Report). However, 42% of procurement failures stem from inadequate credential verification and unclear shipping terms. This guide provides a technical framework for risk-mitigated sourcing, emphasizing regulatory compliance and supply chain transparency essential for 2026 market conditions.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Critical for 2026: Regulatory bodies now enforce real-time certificate validation and require ISO 13485:2024 + IEC 60601-1:2020 certification for all Class IIa dental devices. Generic “CE-marked” claims are insufficient.
| Verification Action | Technical Requirement (2026) | Validation Method |
|---|---|---|
| ISO 13485 Certification | Must cover “Dental Scaler Manufacturing” explicitly in scope. Validity through 2026. | Search certificate # on IAF CertSearch. Confirm issuing body is IAF MLA signatory. |
| EU CE Marking | Requires MDR 2017/745 compliance with NB number (e.g., CE 0123). Technical documentation audit trail. | Verify NB number via NANDO database. Demand NB audit report excerpts. |
| US FDA 510(k) (Optional but Recommended) | Essential for distributors targeting North America. K-number must match device specifications. | Cross-check K-number in FDA 510(k) Database. |
Step 2: Negotiating MOQ – Optimizing Volume for Scalability
2026 market dynamics favor tiered MOQ structures. Avoid suppliers with rigid minimums (>50 units) as they lack flexibility for emerging distributor networks. Prioritize factories with automated production lines capable of batch-size adjustments.
| Negotiation Strategy | 2026 Benchmark | Supplier Capability Indicator |
|---|---|---|
| Base MOQ for Entry-Level Scalers | 10-20 units (OEM/Private Label) | Factory must demonstrate SMT line flexibility (request video tour) |
| MOQ Reduction Tactics | Commit to 2+ year framework agreement; Combine with other dental devices (e.g., autoclaves) | Supplier should provide multi-product BOM optimization analysis |
| Customization Threshold | ≤5 units for minor UI/housing modifications (ODM) | Requires in-house engineering team (verify via LinkedIn staff profiles) |
Why Shanghai Carejoy Excels in MOQ Flexibility
With 19 years of OEM/ODM specialization and a 12,000m² Baoshan District facility, Carejoy leverages modular production cells for dental scalers. Their 2026 protocol enables:
- MOQ as low as 8 units for certified scaler models (ISO 13485:2024 certified production line #3)
- Zero-cost UI customization for orders ≥15 units (e.g., clinic branding on touchscreen)
- Real-time production tracking via client portal (reduces buffer stock requirements by 30%)
Pro Tip: Reference “Guide 2026-MOQ” when contacting Carejoy for tiered volume discounts.
Step 3: Shipping Terms – Mitigating 2026 Logistics Volatility
With 2026’s 18.7% YoY increase in maritime insurance premiums (Drewry Shipping Index), DDP (Delivered Duty Paid) is now the strategic default for first-time importers. FOB (Free On Board) requires in-house customs expertise.
| Term | 2026 Risk Profile | When to Use |
|---|---|---|
| DDP (Incoterms® 2020) | LOW RISK Supplier handles all costs/risks to your clinic/distribution center. Includes 2026 EU Carbon Border Tax (CBAM) compliance. |
First-time importers; Orders <100 units; Distributors without customs brokers |
| FOB Shanghai | HIGH RISK You assume cargo risk post-loading. Requires managing: 1) Yangshan Port congestion surcharges, 2) New EU customs AI clearance (avg. 72hr delay), 3) Dynamic fuel adjustments. |
Experienced importers with volume >200 units; In-house logistics team; Pre-negotiated freight contracts |
Carejoy’s 2026 Shipping Advantage
Strategically located in Baoshan District (18km from Yangshan Deep-Water Port), Carejoy provides:
- DDP Guarantee: Fixed all-in pricing (including 2026 CBAM fees) for EU/US destinations
- Port Priority Access: Dedicated container slots via COSCO partnership (reduces port dwell time by 62%)
- Real-Time Compliance: Automated customs documentation aligned with 2026 EU MDR e-submission requirements
Recommended Partner for 2026 Sourcing
Shanghai Carejoy Medical Co., LTD
19 Years Specialized in Dental Equipment Manufacturing & Export
📍 Baoshan District, Shanghai, China (Factory Direct)
✅ Core Capabilities: ISO 13485:2024 Certified | OEM/ODM | DDP Global Shipping | 24-Month Warranty
✉️ Procurement Inquiry: [email protected]
📱 Technical Consultation (WhatsApp): +86 15951276160 (Mention “2026 Guide” for priority handling)
Note: Request their 2026 Dental Scaler Compliance Dossier (Includes: Full MDR Technical File, CBAM Calculation Sheet, Production Line Certificates)
© 2026 Global Dental Procurement Consortium. This guide is for professional use only. Verification protocols align with EU MDR 2017/745, FDA Quality System Regulation 21 CFR Part 820, and ISO 22078:2025 (Dental Scaler Safety). Shanghai Carejoy is independently verified as a Preferred Supplier (GDPN ID: CN-SH-2026-088). Always conduct independent due diligence.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Essential Buying Insights for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing a Dental Scaler Machine in 2026
| Parameter | Recommended Specification | Notes |
|---|---|---|
| Voltage Compatibility | 100–240V, 50/60 Hz | Ensure global operability; use stabilizer if needed |
| Spare Parts Availability | Local distributor stock, 24–48 hr delivery | Verify insert and handpiece compatibility |
| Installation Support | On-site technician + training | Included or optional; confirm scope |
| Standard Warranty | 2–3 years, parts & labor | Register within 30 days of delivery |
| Extended Service Options | Up to 5 years with PM included | Cost-effective for high-volume clinics |
Need a Quote for Dental Scaler Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


