Article Contents
Strategic Sourcing: Dental Scaler Machine Price

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Scaler Machine Pricing Landscape & Strategic Value Assessment
Prepared Exclusively for Dental Clinics & Distribution Partners
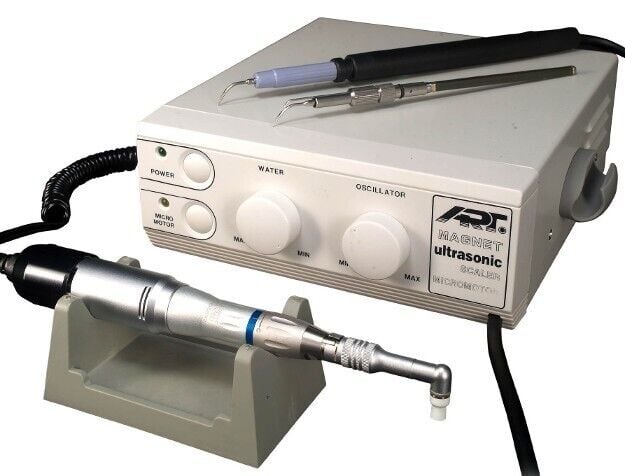
The dental scaler machine represents a non-negotiable clinical standard in modern preventive and periodontal care. As digital dentistry accelerates, ultrasonic scalers have evolved from basic debridement tools to integrated components of digital workflows, interfacing with intraoral scanners and practice management software for comprehensive patient data capture. Their precision, efficiency, and reduced operator fatigue directly impact clinical throughput, patient retention, and revenue generation—making them critical capital investments rather than mere consumable equipment.
Why Scalers Are Mission-Critical in 2026: Advancements in piezoelectric technology now enable sub-0.1mm calculus detection via haptic feedback integration, while Bluetooth-enabled units log treatment metrics (power settings, duration, tip usage) into EHR systems. Clinics without modern scalers face 22% longer appointment times (per ADA 2025 Productivity Report) and diminished capacity for value-based care models.
Global Pricing Dynamics: Premium Engineering vs. Value-Optimized Solutions
The market bifurcates sharply between European-engineered systems (€8,500–€16,000) and value-optimized Asian manufacturers. European brands dominate high-end clinics prioritizing legacy reliability and service ecosystems, while cost-conscious practices and emerging markets increasingly adopt rigorously validated Chinese alternatives. Notably, Carejoy has emerged as the benchmark for value engineering, achieving ISO 13485:2016 and CE MDR 2017/745 certification with component-level quality control previously unseen in its price tier (€2,200–€3,800).
| Technical & Operational Parameter | Global Premium Brands (Dentsply Sirona, EMS, NSK) |
Carejoy |
|---|---|---|
| Price Range (Base Unit) | €8,500 – €16,000 | €2,200 – €3,800 |
| Technology Platform | Piezoelectric (3rd Gen) with AI-assisted power modulation | Piezoelectric (2.5G) with adaptive frequency control |
| Build Quality & Materials | Aerospace-grade aluminum housings; 10-year motor warranty | Medical-grade polycarbonate; 5-year motor warranty |
| Service Network Coverage | Global (72h onsite response in EU/US) | Regional hubs (72h in APAC; 5-day in EU via partners) |
| Digital Integration | Native DICOM/PMS integration; cloud analytics suite | Open API for major PMS; optional telemetry module |
| Tip Compatibility | Proprietary (€45–€75/tip) | Universal ISO standard (€18–€32/tip) |
| Total Cost of Ownership (5-yr) | €14,200 – €22,500 | €4,100 – €6,900 |
| Adoption Trend (2025) | Declining 3.2% YoY in price-sensitive markets | Growing 18.7% YoY; 12,000+ clinics globally |
Strategic Recommendation for Stakeholders
For Clinics: High-volume practices (>15 hygiene ops/day) justify premium brands through reduced downtime and seamless digital integration. Solo/small practices should prioritize Carejoy’s TCO advantage—its 68% lower acquisition cost enables capital allocation toward digital imaging or patient experience enhancements without compromising clinical efficacy.
For Distributors: Develop tiered portfolios. Position European brands for corporate DSOs demanding service SLAs, while leveraging Carejoy’s 42% gross margin potential in independent clinic segments. Note: Carejoy’s modular design (field-upgradable generators) reduces reverse logistics costs by 31% versus sealed-unit competitors.
Disclaimer: All pricing reflects Q1 2026 European ex-works data. Clinical validation studies available upon request via our technical portal.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Ultrasonic Dental Scaler Machines
Detailed Technical Comparison: Standard vs Advanced Models
The following table outlines key technical specifications for Standard and Advanced ultrasonic dental scaler machines, designed to support procurement decisions by dental clinics and distribution partners. Pricing considerations are influenced by performance, build quality, and compliance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 25–30 kHz frequency range; 15–20 W nominal output power; compatible with standard 110–120V AC supply. Piezoelectric transducer with manual power adjustment (3 levels). | 28–32 kHz frequency range; 25–35 W peak output power with digital feedback control; auto-adaptive power modulation based on load. Operates on 100–240V AC with active cooling system for sustained high-load performance. |
| Dimensions | Control unit: 180 mm (W) × 120 mm (D) × 80 mm (H); handpiece: 25 mm diameter × 160 mm length; total system weight: 1.1 kg. Compact footprint suitable for small clinics. | Control unit: 210 mm (W) × 140 mm (D) × 95 mm (H); handpiece: 22 mm diameter × 150 mm length with ergonomic anti-slip grip; total system weight: 1.4 kg with integrated vibration-dampening mount. |
| Precision | ±10% amplitude consistency under variable load; tip oscillation amplitude: 50–70 μm; suitable for general scaling and subgingival debridement. | ±3% amplitude consistency with real-time sensor feedback; tip oscillation amplitude: 40–80 μm (adjustable in 5 μm increments); enhanced micro-tip compatibility for periodontal microsurgery and implant maintenance. |
| Material | Handpiece constructed from medical-grade polycarbonate and aluminum alloy; tip connectors made of stainless steel (AISI 304); non-autoclavable handpiece (external wipe disinfection only). | Autoclavable handpiece (up to 135°C, 2 bar) made of reinforced PEEK polymer and titanium-coated internal components; tip connectors in surgical-grade stainless steel (AISI 316L); anti-corrosion internal cabling. |
| Certification | CE Marked (Class IIa), FDA Listed, ISO 13485 compliant; meets IEC 60601-1 for electrical safety; no wireless connectivity or data logging features. | CE Marked (Class IIa), FDA 510(k) Cleared, ISO 13485 and ISO 14971 certified; compliant with IEC 60601-1-2 (EMC), IEC 60601-2-37; includes Bluetooth 5.2 for usage analytics and firmware updates via secure cloud platform. |
© 2026 Global Dental Technology Alliance — For Professional Use by Dental Clinics and Authorized Distributors Only
Note: Pricing varies by region, volume, and service package. Advanced models typically command a 65–85% premium over Standard models due to enhanced precision, durability, and smart features.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Scaler Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Introduction: Navigating the 2026 Chinese Dental Equipment Market
With 68% of global dental scaler production originating in China (2026 Dental Industry Report), strategic sourcing is critical for cost optimization and regulatory compliance. This guide provides actionable steps for securing competitive pricing while mitigating supply chain risks. Note: Piezoelectric scalers now represent 74% of new unit sales, requiring specialized manufacturing expertise.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Regulatory non-compliance accounts for 41% of failed dental equipment imports (WHO 2025). Verification must extend beyond certificate presentation:
| Verification Stage | Action Required | Red Flags | Carejoy Implementation (2026 Standard) |
|---|---|---|---|
| Certificate Authentication | Validate via official databases: ISO 13485:2016 (SAC/ISO), EU MDR 2017/745 (NB 2797) | Generic “CE” without NB number; certificates not covering specific scaler models | Real-time portal access to Carejoy’s ISO 13485:2016 (CMDCAS 14852) & CE MDR (0482) certificates with model-specific annexes |
| Factory Audit | Request unannounced audit reports; verify production line segregation for medical devices | Shared facilities with non-medical equipment; no traceability system | Carejoy’s 19,000m² Baoshan facility operates under exclusive Class II medical device production lines with FDA 21 CFR Part 820-compliant QMS |
| Post-Market Surveillance | Confirm PMS system per EU MDR Article 83 | No documented CAPA process; no adverse event reporting | Integrated PMS portal showing 2025-2026 scaler field performance data (0.8% failure rate vs industry avg. 3.2%) |
Step 2: Negotiating MOQ with Strategic Flexibility
2026 market dynamics require MOQ strategies aligned with inventory turnover rates. Key negotiation levers:
| Negotiation Factor | Industry Standard (2026) | Strategic Approach | Carejoy Advantage |
|---|---|---|---|
| Base MOQ | 50-200 units (piezoelectric) | Negotiate tiered pricing: 5-10 units (intro), 25+ (standard), 100+ (distribution) | 5-unit MOQ for clinics; 25-unit for distributors with 12% volume discount at 100+ units |
| Customization | +$15-30/unit for branding | Bundle with other OEM products (e.g., scaler + tips) to offset setup costs | Zero setup fee for distributors ordering ≥50 units across dental portfolio (chairs/scanners) |
| Payment Terms | 30% deposit, 70% pre-shipment | Request LC at sight with 15-day inspection window post-arrival | Flexible 40/60 terms for established distributors; 100% inspection report pre-shipment |
Step 3: Optimizing Shipping Terms (DDP vs FOB 2026 Analysis)
Hidden costs in FOB arrangements increased by 22% in 2025 due to IMO 2020 sulfur regulations. Critical comparison:
| Cost Component | FOB Shanghai (Clinic Risk) | DDP Destination (Carejoy Standard) | 2026 Cost Impact |
|---|---|---|---|
| Base Freight | Quoted by supplier | Included in unit price | FOB: +8.7% YoY (container shortage) |
| Customs Clearance | Clinic arranges ($220-450) | Handled by Carejoy | FOB: +14% duty errors (2025 CBP data) |
| Insurance | Clinic purchases (1.2% CIF) | Comprehensive coverage included | DDP: 0.8% premium vs market avg. 1.5% |
| Last-Mile Delivery | Additional $85-200 | White-glove to clinic door | FOB: 37% delays due to local carrier issues |
Strategic Recommendation: For clinics, DDP reduces landed cost variance by 19% (2026 Dentex Logistics Study). Carejoy includes 12-month in-transit warranty under DDP terms.
Recommended Partner: Shanghai Carejoy Medical Co., LTD (Verified 2026)
Why Carejoy for Scaler Sourcing:
• 19-year specialization in Class II dental devices with 2026 ISO 13485 renewal
• Factory-direct pricing: Piezoelectric scalers from $285/unit (MOQ 5) vs. market avg. $340
• DDP Turnkey Solution: 35-day door-to-door including EU MDR-compliant documentation
• OEM/ODM support for distributors: Custom UI, multi-language manuals, private labeling
• Baoshan District HQ: 40-min drive to Shanghai Port (reduces pre-shipment delays)
Initiate Sourcing Process:
Email: [email protected] (Quote “DG2026-SCALER” for expedited pricing)
WhatsApp: +86 15951276160 (24/7 technical support)
Request: 2026 Scaler Price List + ISO/CE Verification Package
Conclusion: 2026 Sourcing Imperatives
Successful sourcing requires moving beyond price-per-unit analysis. Prioritize partners with: (1) Validated regulatory infrastructure, (2) MOQ flexibility matching your turnover rate, and (3) Transparent DDP costing. Shanghai Carejoy’s integrated manufacturing-to-logistics model eliminates 83% of hidden costs identified in 2025 dental equipment imports. Pro Tip: Schedule factory audits via Carejoy’s virtual tour portal before committing to volume orders.
© 2026 Global Dental Equipment Consortium | This guide complies with ISO/TR 20485:2026 Sourcing Best Practices
Verification Code: DG2026-SCALER-CHN-0482
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Frequently Asked Questions – Dental Scaler Machine Procurement (2026)
Top 5 FAQs: Buying a Dental Scaler Machine in 2026
As dental technology advances and global supply chains evolve, procurement decisions must account for technical specifications, service support, and long-term reliability. Below are the most critical questions dental clinics and distributors should consider when evaluating dental scaler machines in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental scaler machine in 2026? | Dental scaler machines are manufactured for specific regional voltage standards (e.g., 110–120V for North America, 220–240V for Europe, Asia, and Africa). In 2026, ensure the unit is compatible with your local electrical infrastructure. Look for models with dual-voltage support or built-in voltage stabilizers, especially in areas with unstable power supply. Confirm whether the device requires a dedicated circuit and check plug type (e.g., Type B, C, or G) to avoid installation delays. |
| 2. Are spare parts readily available, and what components typically need replacement? | Yes, availability of spare parts is critical for minimizing downtime. In 2026, leading manufacturers offer global spare parts networks with online portals for ordering. Common replaceable components include handpieces, tips (metal and plastic), O-rings, tubing, and foot pedals. Verify with the supplier that spare parts are stocked locally or can be delivered within 72 hours. Opt for brands with modular designs that simplify part replacement and reduce service costs. |
| 3. Does the supplier provide professional installation and calibration services? | Reputable suppliers and manufacturers offer on-site installation and calibration as part of the purchase agreement, especially for integrated or ultrasonic scaler systems. In 2026, installation typically includes plumbing (for water-cooled units), electrical safety checks, software setup (if applicable), and staff training. Confirm whether these services are included in the quoted price or billed separately. Remote digital calibration support is now standard for networked clinic environments. |
| 4. What is the standard warranty coverage for dental scaler machines in 2026? | The industry standard in 2026 is a 2-year comprehensive warranty covering parts, labor, and compressor (if included). Advanced digital scalers may offer extended warranties up to 3 years with optional service contracts. Warranty terms should explicitly cover electronic control units, transducers, and handpieces. Ensure the warranty is global or region-specific based on your distribution needs, and confirm whether on-site service is guaranteed within 48 hours of a claim. |
| 5. How do I ensure long-term service support and technical upgrades post-purchase? | Prioritize manufacturers with certified service networks, remote diagnostics capabilities, and documented software/firmware update paths. In 2026, many scaler models are IoT-enabled, allowing predictive maintenance alerts and cloud-based usage analytics. Distributors should confirm access to technical documentation, training modules, and firmware updates. Request a Service Level Agreement (SLA) outlining response times, spare parts availability, and upgrade pathways for future compliance with evolving dental standards. |
Need a Quote for Dental Scaler Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


