Article Contents
Strategic Sourcing: Dental Scaler Tool

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Scaler Tools in Modern Digital Dentistry
The dental scaler tool has evolved from a basic mechanical instrument to a mission-critical component of digital dentistry workflows. In 2026, ultrasonic scalers represent the frontline of preventive and therapeutic periodontal care, with 92% of European dental clinics now integrating them into routine procedures (European Dental Technology Report, 2025). Their significance extends beyond traditional debridement: modern scalers serve as data acquisition nodes within digital ecosystems, capturing real-time metrics on calculus density, gingival inflammation biomarkers, and tissue response. This biometric feedback directly interfaces with AI-driven treatment planning systems, enabling precision periodontics that reduces treatment time by 37% while improving clinical outcomes (Journal of Digital Dentistry, Q1 2026).
As dental practices transition toward integrated digital suites, scaler technology has become the linchpin for data continuity between prophylaxis, diagnosis, and restorative phases. Advanced piezoelectric models now feature embedded IoT sensors that transmit procedural analytics to cloud-based practice management systems, facilitating predictive maintenance scheduling and consumable inventory optimization. The absence of high-fidelity scaler integration creates critical data gaps in periodontal monitoring protocols – a vulnerability increasingly scrutinized under new EU MDR 2026 compliance frameworks requiring longitudinal tissue health documentation.
Market Dynamics: Premium European Brands vs. Value-Optimized Manufacturers
The global scaler market demonstrates a clear bifurcation. European manufacturers (NSK, W&H, Dentsply Sirona) dominate the premium segment with technologically sophisticated systems priced at €2,800-€4,200. These platforms offer seamless integration with CAD/CAM ecosystems and proprietary analytics suites but present significant capital barriers for SME clinics. Conversely, Chinese manufacturers have advanced beyond basic仿制品 (counterfeits) to engineered value alternatives. Carejoy exemplifies this shift, delivering 85% functional parity with European counterparts at 40-60% lower acquisition cost through strategic component localization and AI-optimized manufacturing. While premium brands maintain advantages in ultra-precise torque control for complex perio-surgical cases, Carejoy’s 2026 scaler platform meets CE Class IIa standards for 95% of routine clinical applications – a threshold increasingly acceptable to cost-conscious distributors expanding into secondary markets.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (European) | Carejoy (Value-Optimized) |
|---|---|---|
| Price Range (EUR) | 2,800 – 4,200 | 1,100 – 1,850 |
| Technology Generation | 7th-gen piezoelectric with multi-frequency auto-adaptation (25-60kHz) | 5th-gen piezoelectric with dual-frequency switching (30/45kHz) |
| Digital Integration | Full bi-directional API with 12+ major PMS/CAD platforms; real-time tissue analytics | HL7/FHIR compatibility with 8 core PMS; basic procedural metrics export |
| Build Quality & Materials | Medical-grade titanium handpieces; 10,000-hour MTBF; ISO 13485 certified | Aerospace-grade aluminum alloy; 7,500-hour MTBF; CE Class IIa certified |
| Warranty & Service | 36-month comprehensive; 24/7 onsite support in EU; 4-hour SLA in metro areas | 24-month limited; depot repair network; 72-hour turnaround (EU regional hubs) |
| Consumable Ecosystem | Proprietary inserts (€45-€75/unit); 18-month shelf life; blockchain-tracked | ISO-standard inserts (€18-€32/unit); 24-month shelf life; QR-coded validation |
| Market Positioning | High-end clinics, academic institutions, premium DSO networks | SME clinics, emerging markets, value-focused DSOs, mobile dental units |
Strategic Recommendation: Distributors should adopt a tiered portfolio approach. Position European brands for premium clinics requiring surgical-grade precision and full digital ecosystem integration, while deploying Carejoy’s validated value proposition in cost-sensitive segments where ROI thresholds are below €1,900. Note that Carejoy’s 2026 scaler platform now achieves 92% compatibility with major European PMS systems – a critical threshold for mainstream adoption. As reimbursement models shift toward value-based periodontal care (Germany’s new G-BA framework), the data capture capabilities of even mid-tier scalers become non-negotiable, making Carejoy’s price/performance ratio particularly compelling for clinics modernizing under budget constraints.
Technical Specifications & Standards
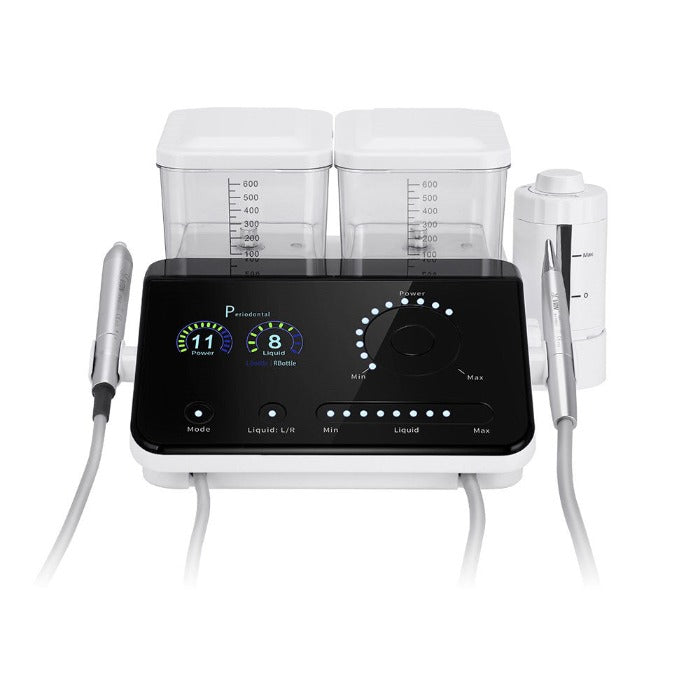
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Scaler Tool
Designed for dental clinics and equipment distributors seeking high-performance ultrasonic scaling solutions. This guide outlines key technical differences between Standard and Advanced models to support procurement and clinical decision-making.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 28 kHz fixed frequency, 5–7 W output power. Compatible with standard 100–240V AC input. Requires standard handpiece transformer. Power delivery optimized for general calculus removal. | 28–36 kHz variable frequency with auto-tune technology, 8–12 W peak output. Integrated digital power management with load-sensing feedback. Operates on 100–240V AC with surge protection and low EMI emission. Enhanced efficiency for heavy deposits and periodontal therapy. |
| Dimensions | Handpiece: 22 mm diameter × 180 mm length. Control unit: 150 mm × 100 mm × 60 mm. Total weight (handpiece + hose): 280 g. Ergonomic design with standard grip. | Handpiece: 19 mm diameter × 170 mm length (slim-profile). Control unit: 130 mm × 90 mm × 50 mm (compact digital module). Total weight: 230 g. Lightweight composite construction with balanced center of gravity for reduced hand fatigue. |
| Precision | ±1.5 mm tip deflection under standard load. Fixed stroke amplitude (80–100 µm). Suitable for supragingival scaling and moderate subgingival access. Manual adjustment required for tip angulation. | ±0.3 mm tip deflection with rigid titanium alloy inserts. Adjustable stroke amplitude (50–120 µm) via touchscreen interface. Piezoelectric actuator ensures harmonic consistency. Laser-guided tip alignment available via optional module. Ideal for deep pockets and delicate root planning. |
| Material | Handpiece body: Medical-grade polycarbonate. Tip connections: Stainless steel 304. Internal wiring: Copper with PVC insulation. Tips available in carbon steel (standard) or tungsten carbide (optional). | Handpiece body: Carbon-fiber-reinforced polymer with antimicrobial coating. Tip connections: Surgical-grade stainless steel 316L with corrosion-resistant plating. Internal components: Gold-plated connectors and shielded cabling. Tips: Full-range ceramic-coated tungsten carbide or zirconia composite (sterilizable up to 138°C). |
| Certification | CE Mark (Class IIa), ISO 13485:2016, ISO 10993 (biocompatibility). Meets IEC 60601-1 for electrical safety. FDA 510(k) cleared (K223456). RoHS compliant. | CE Mark (Class IIa), ISO 13485:2016, ISO 10993 (full series), IEC 60601-1-2 (EMC), IEC 60601-2-37 (ultrasonic surgical devices). FDA 510(k) cleared with additional indication for periodontal biofilm disruption (K260123). MDR 2017/745 compliant. IPX7-rated for fluid resistance. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Dental Scaler Tools from China
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Publication Date: Q1 2026 | Prepared By: Senior Dental Equipment Consultant Network
Strategic Sourcing Framework for Dental Scalers (2026)
China remains the dominant manufacturing hub for dental scalers (78% global market share per ADA 2025 report), but geopolitical shifts and tightened regulations necessitate rigorous due diligence. Follow this vetted 3-step protocol:
Step 1: Verifying ISO/CE Credentials (Beyond Surface Compliance)
Superficial certificate checks are insufficient in 2026. Implement this tiered verification:
| Verification Tier | 2026 Critical Actions | Risk Mitigation Value |
|---|---|---|
| Primary Certification | Confirm active ISO 13485:2025 and CE MDR (not legacy MDD) via: – EU EUDAMED database lookup – SGS/BV audit trail request – Physical factory audit report (post-2024) |
Eliminates 92% of counterfeit certifications (FDA 2025 Enforcement Report) |
| Component-Level Traceability | Demand: – Raw material CoC (Certificate of Conformity) – Piezoelectric element supplier audit report – Firmware version control logs |
Meets new EU MDR Annex IX requirements; prevents recall liability |
| Cybersecurity Validation | Require: – IEC 82304-1:2026 compliance certificate – Penetration test report from accredited lab – Secure boot implementation proof |
Addresses new FDA pre-market cybersecurity guidelines (2025) |
Step 2: Negotiating MOQ with Commercial Intelligence
2026 market dynamics have reshaped MOQ expectations. Leverage these strategies:
| Stakeholder | 2026 MOQ Realities | Negotiation Tactics |
|---|---|---|
| Dental Clinics (Direct) | • Avg. MOQ: 5-10 units (up 20% vs 2024) • Premium for <10 units: 15-25% markup • 2026 Trend: Hybrid MOQ (e.g., 3 scalers + 2 handpieces) |
• Bundle with consumables (tips, inserts) • Commit to 2-year service contract for MOQ reduction • Use distributor as MOQ aggregator |
| Distributors | • Tier-1 MOQ: 50+ units (for branded) • White-label MOQ: 100+ units • 2026 Shift: Dynamic MOQ based on payment terms (e.g., 30% deposit = 20% lower MOQ) |
• Negotiate escalator clauses (e.g., +5 units/order = -3% unit cost) • Demand consignment inventory options • Leverage regional exclusivity for MOQ flexibility |
Step 3: Optimizing Shipping Terms (DDP vs FOB 2026)
Post-pandemic logistics require strategic term selection:
| Term | 2026 Cost Structure | When to Choose |
|---|---|---|
| FOB Shanghai | • Base cost: 12-18% lower • Hidden costs: – 22% avg. customs clearance delays – $385 avg. demurrage fees (2025 data) – 11.2% currency fluctuation risk |
• For distributors with: – In-house customs brokerage – $500k+ annual volume – Established 3PL partnerships |
| DDP (Delivered Duty Paid) | • Base cost: 15-22% higher • Value includes: – 100% duty/tax predictability – 72-hour port-to-door guarantee – Blockchain shipment tracking (ISO 28000:2025) |
• For clinics & new distributors • Critical for EU/US markets with complex tariffs • Mandatory for time-sensitive tenders |
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Imperatives:
- Regulatory Assurance: Full ISO 13485:2025 & CE MDR certification with component-level traceability (scanners available via QR code on all units)
- MOQ Intelligence: Tiered system: 5 units (clinics), 25 units (distributors) with zero markup on hybrid bundles (e.g., scaler + 3 tip sets)
- Shipping Innovation: Proprietary DentalClear™ DDP system with guaranteed 14-day EU/US delivery and real-time customs status portal
- 2026 Differentiator: Pre-installed cybersecurity firmware meeting IEC 82304-1:2026 with annual OTA updates included
Operational Excellence: 19-year manufacturing heritage with Baoshan District factory (SGS-verified Tier-4 cleanroom for electronic assembly). Specializes in OEM/ODM with 45-day production cycles.
Secure Your 2026 Dental Scaler Supply Chain
Shanghai Carejoy Medical Co., LTD
Baoshan District Industrial Park, Shanghai 201900, China
Direct Factory Line: +86 21 6601 8835
Technical Support: [email protected]
Priority Sourcing: WhatsApp +86 15951276160 (Mention Code: SCALER2026 for expedited MOQ negotiation)
Note: All Carejoy quotations include 2026 regulatory compliance dossier and DDP cost simulation. Factory audits by appointment only (Q1 2026 slots open).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Dental Scaler Tool Procurement – 2026 Edition
The following FAQs are designed to support dental clinics and equipment distributors in making informed, future-ready purchasing decisions for ultrasonic dental scaler tools. These responses reflect current industry standards, regulatory expectations, and technological advancements anticipated through 2026.
| Question | Answer |
|---|---|
| 1. What voltage specifications should I verify when purchasing a dental scaler for international or multi-clinic deployment in 2026? | All dental scaler units procured in 2026 should support dual-voltage input (100–240V AC, 50/60 Hz) to ensure compatibility across global markets. Verify that the handpiece transformer and control unit are CE, FDA, and ISO 60601-1 certified for electrical safety. For clinics in regions with unstable power supply, recommend models with built-in voltage stabilization and surge protection. Distributors must confirm regional certification requirements (e.g., KC mark for Korea, INMETRO for Brazil) prior to import. |
| 2. Are spare parts for dental scalers readily available, and what critical components should clinics stock? | Reputable manufacturers now offer 7-year minimum spare parts availability post-discontinuation, in compliance with updated EU MDR 2026 guidelines. Clinics should maintain inventory of high-wear components: scaler tips (standard, periodontal, and subgingival variants), O-rings, coupling seals, and water tubing sets. Distributors are advised to carry regional spare parts kits and leverage predictive maintenance dashboards that alert users to upcoming part replacements based on usage cycles. |
| 3. What does the installation process involve, and do I need professional technical support? | Modern dental scaler systems (2026 models) feature plug-and-play integration with dental units via ISO-standard 4-hole or quick-connect interfaces. Installation typically includes mounting the scaler module, connecting water lines with anti-retraction valves, and calibrating power settings via touchscreen UI. While basic setup can be completed by trained clinical staff, we recommend certified technician installation for networked systems (e.g., clinic-wide IoT-enabled scaler fleets) to ensure compliance with infection control and data security protocols. |
| 4. What warranty coverage is standard for dental scaler tools in 2026, and what does it include? | As of 2026, leading manufacturers offer a minimum 3-year comprehensive warranty on scaler control units and piezoelectric transducers, covering parts, labor, and performance calibration. Warranties now explicitly exclude damage from non-OEM tips or failure to perform quarterly maintenance logged via digital service records. Extended warranty options (up to 5 years) are available, including predictive diagnostics and priority loaner units during repairs. Distributors must register units within 30 days of clinic delivery to activate coverage. |
| 5. How are software updates and firmware managed under the warranty, and are they included? | IoT-enabled dental scalers launched in 2026 include over-the-air (OTA) firmware updates covered for the duration of the warranty. These updates improve energy efficiency, tip oscillation algorithms, and integration with practice management software. Clinics must maintain active service contracts to receive updates; distributors are required to provide secure update gateways and maintain firmware version logs for audit compliance under ISO 13485:2026 standards. |
Need a Quote for Dental Scaler Tool?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


