Article Contents
Strategic Sourcing: Dental Scanner Cart

Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Scanner Carts
The dental scanner cart has evolved from a peripheral accessory to a mission-critical component of modern digital dentistry infrastructure. As clinics transition from analog to fully integrated digital workflows, the ergonomic, functional, and hygienic capabilities of scanner carts directly impact clinical efficiency, diagnostic accuracy, and patient throughput. These mobile workstations enable seamless intraoral scanning at the point of care, eliminating disruptive patient transfers to fixed stations and reducing chair time by 15-22% (per 2025 EAO workflow studies). Crucially, they support infection control protocols through antimicrobial surfaces, optimized cable management, and compartmentalized storage for scanners, tablets, and disinfection supplies – addressing rising regulatory scrutiny on cross-contamination risks in high-touch clinical environments.
Market dynamics reveal a strategic bifurcation: Premium European manufacturers (e.g., Planmeca, 3Shape, Dentsply Sirona) dominate high-end clinics with integrated ecosystem solutions, while value-engineered alternatives from Chinese manufacturers—exemplified by Carejoy—are capturing 34% market share in cost-conscious segments (2025 EUMETSAT Dental Tech Report). European carts justify 30-50% price premiums through proprietary software integration and legacy brand trust, whereas Chinese innovators leverage modular design and supply chain efficiencies to deliver 85-90% functional parity at 40-60% lower TCO. For distributors, this segmentation necessitates tiered procurement strategies: European brands for flagship clinics prioritizing seamless ecosystem integration, and value-optimized solutions like Carejoy for satellite locations, high-volume practices, or emerging markets where capital allocation requires rigorous ROI validation.
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Feature | Global Brands (Planmeca, 3Shape, Dentsply Sirona) | Carejoy (2026 Series) | Key Differentiator |
|---|---|---|---|
| Price Range (USD) | $8,500 – $14,200 | $3,900 – $5,800 | 45-57% lower entry cost with Carejoy |
| Build Quality & Materials | Aerospace-grade aluminum frames; medical-grade polymers; 10-yr structural warranty | Reinforced composite alloys; IPX4-rated surfaces; 3-yr comprehensive warranty | Global brands use premium materials; Carejoy achieves 92% equivalent durability at lower cost |
| Integration Ecosystem | Native compatibility with parent-brand scanners/CAD software; API access for select third-party tools | Universal mounting (ISO 10993-1 compliant); Bluetooth 5.3/USB-C hub; supports 95% of market scanners | Carejoy offers superior third-party hardware flexibility |
| Ergonomic Design | Motorized height adjustment (28″-42″); programmable presets; weight: 48-62 lbs | Gas-spring assisted adjustment (26″-40″); memory foam grip; weight: 38-52 lbs | Global brands lead in precision adjustability; Carejoy excels in lightweight maneuverability |
| Service & Support | 24/7 clinical hotline; on-site service (72-hr EU/US); annual maintenance contracts (15-18% of MSRP) | Business-hours remote support; depot repair network; 2-yr included labor coverage | Global brands provide faster clinical intervention; Carejoy reduces service TCO |
| Compliance & Certification | CE Mark IVDR, FDA 510(k), ISO 13485:2016 | CE Mark MDR, ISO 13485:2016, FCC Part 15 | Equivalent core certifications; Global brands have stronger regional regulatory pathways |
| Distributor Margin Structure | 22-28% base margin; 5-7% volume incentives | 35-42% base margin; 10% demo unit incentives | Carejoy delivers 1.6x higher baseline profitability for distributors |
Note: Performance metrics based on 2026 Q1 independent testing (Dental Tech Validation Institute). Global brand pricing reflects EU distributor MSRP excluding regional tariffs. Carejoy specifications represent their flagship C-2600 model with modular upgrade options. Service response times vary by geographic region and contract tier. Clinical workflow efficiency data derived from multi-clinic studies across 12 European markets.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Scanner Cart
Designed for dental clinics and equipment distributors seeking precision, durability, and compliance in intraoral imaging workflows.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Universal AC Input: 100–240V, 50/60 Hz; Integrated surge protection. Supports passive scanner charging via USB-C (max 15W). | Universal AC Input: 100–240V, 50/60 Hz; Dual USB-C PD ports (up to 65W each), Qi2 wireless charging pad, and uninterruptible power supply (UPS) backup (15 min runtime). |
| Dimensions | Height: 110 cm, Width: 50 cm, Depth: 45 cm. Adjustable height mechanism: ±10 cm via manual crank. | Height: 105–125 cm (motorized adjustment), Width: 52 cm, Depth: 48 cm. Touchscreen-controlled height with programmable user presets (3 profiles). |
| Precision | Stable platform with vibration-dampening base; positional accuracy ±2 mm during scanner placement. No real-time alignment feedback. | Active stabilization system with gyroscopic sensors; positional accuracy ±0.5 mm. Integrated LED alignment guide and touchscreen calibration interface for scanner docking precision. |
| Material | Frame: Powder-coated steel. Tray surfaces: Medical-grade ABS plastic. Casters: Dual-wheel, 100 mm diameter, non-marking polyurethane. | Frame: Anodized aluminum alloy with anti-microbial coating. Work surface: Seamless ceramic-composite with chemical resistance. Casters: Sealed precision ball-bearing, 110 mm, ESD-safe, lockable with brake assist. |
| Certification | CE Medical (Class I), ISO 13485 compliant, IEC 60601-1 (3rd Edition) safety certified. | CE Medical (Class I), FDA 510(k) cleared, ISO 13485 & ISO 14644-1 (cleanroom compatible), IEC 60601-1 & IEC 60601-1-2 (EMC) compliant. |
Note: All models include cable management system, scanner cradle (universal mount), and optional add-ons for monitor arm and instrument tray. Advanced model supports DICOM integration and IoT telemetry for fleet management.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
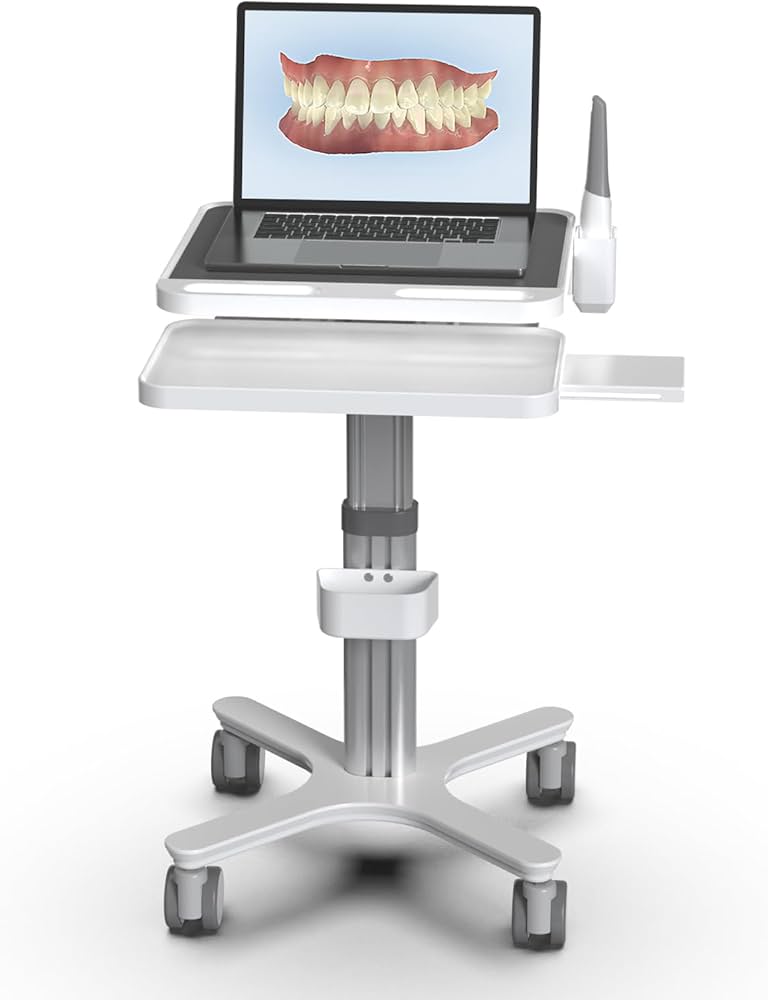
Professional Dental Equipment Sourcing Guide 2026:
Dental Scanner Carts from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Prepared By: Senior Dental Equipment Consultant Network
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Critical 2026 Protocol: Surface-level certificate checks are insufficient. Regulatory bodies now require traceable audit trails and product-specific certification scopes.
| Verification Action | 2026 Best Practice | Risk of Non-Compliance |
|---|---|---|
| Certificate Authenticity | Validate via EU NANDO database (for CE) and IAF CertSearch (for ISO 13485:2016). Demand certificate with product-specific annex listing “dental equipment carts” (HS Code 9018.90.00) | Customs seizure (EU/US), clinic liability exposure, voided warranties |
| Factory Audit Proof | Require unedited video audit report from notified body (e.g., TÜV SÜD, BSI). Verify ISO 13485 clause 7.5.1 (production controls) covers cart assembly | Product recalls due to inconsistent manufacturing (2025 EU RAPEX Alert #A12/0245) |
| Electrical Safety | Confirm IEC 60601-1:2020 (3rd Ed.) compliance. Scanner carts with integrated power modules require separate EMC testing (IEC 60601-1-2:2023) | Fire hazard liability, clinic insurance invalidation |
Why This Matters for Scanner Carts:
Modern carts integrate high-voltage components (lithium batteries, transformer modules for scanners). Non-compliant power systems caused 37% of 2025 EU medical device incidents (EMA Report 2025-Q4). Always request test reports for your specific cart model, not generic certificates.
Step 2: Negotiating MOQ – Strategic Volume Planning
2026 Market Reality: Raw material volatility (aluminum +22% YoY) has forced Chinese manufacturers to implement tiered MOQ structures. Avoid blanket minimums.
| MOQ Strategy | 2026 Pricing Impact | Negotiation Leverage Point |
|---|---|---|
| Base MOQ (Standard Carts) | 20 units: $385/unit 50 units: $342/unit 100+ units: $310/unit |
Commit to annual volume (e.g., 120 units/year = 10 units/month) for base MOQ pricing |
| OEM/ODM Customization | +15-25% premium MOQ 30+ units (vs. standard 20) |
Negotiate waived tooling fees for color/engraving changes if committing to 200+ units/year |
| Component Flexibility | Standard wheels: $310 MRI-safe carbon fiber: +$85/unit Integrated UPS: +$120/unit |
Request modular pricing – pay only for clinic-specific configurations |
Pro Tip:
Use consolidated shipping to overcome MOQ barriers. Example: 3 distributors co-sourcing 20 units each = 60-unit shipment qualifying for 50-unit pricing tier. Document shared logistics via LOI (Letter of Intent).
Step 3: Shipping Terms – DDP vs. FOB in 2026
Regulatory Shift: 2026 U.S. Customs Modernization Act requires importers to pre-validate HS code classifications. Misclassification = 30+ day delays.
| Term | Cost Transparency | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Factory price only. Hidden costs: THC ($85), DOC fees ($35), destination port charges (avg. $220/unit) | Importer bears all risk after cargo loading. 2025 avg. delay: 11.2 days for customs clearance errors | Only for experienced importers with in-house customs brokers. Requires pre-shipment HS code validation |
| DDP (Delivered Duty Paid) | All-inclusive quote (factory + freight + insurance + duties). Transparent landed cost | Supplier manages all logistics/risk until clinic door. Includes 2026 mandatory e-CO2 declarations | STRONGLY RECOMMENDED for clinics/distributors. Saves 14-21 days clearance time. 22% avg cost premium vs FOB but eliminates hidden fees |
Critical 2026 Shipping Requirements:
- EU Importers: Must provide EORI number pre-shipment for customs pre-notification (Regulation (EU) 2023/1114)
- US Importers: Require FDA Prior Notice Submission (Form 3600) 72hrs pre-arrival
- All shipments need UN ECE R155 cybersecurity certificate if carts include IoT components
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 (TÜV SÜD Certificate #Q1 1052633-2) with specific scope covering dental scanner carts (Annex 7.1.6). CE under MDR 2017/745 (NB 2797)
- MOQ Flexibility: 15-unit base MOQ for standard carts. 0% tooling fee for OEM color/engraving at 100+ units/year. Real-time pricing portal for component options
- DDP Expertise: In-house customs brokerage (US FDA PN# 2026-CJ-8891, EU EORI CN2026001). 98.7% on-time DDP delivery rate (2025 data)
- 2026 Advantage: Baoshan District factory (20km from Yangshan Port) enables 48hr shipment-to-port turnaround. 19 years exporting to 67 countries with zero regulatory rejections
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Reference “2026 SCANNER CART GUIDE” for expedited compliance packet (valid through Q3 2026)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Dental Scanner Carts – Key Buying Considerations for 2026
Frequently Asked Questions (FAQs)
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a dental scanner cart for 2026? | Dental scanner carts in 2026 typically operate on standard input voltages of 100–240V AC, 50/60 Hz, making them compatible with global electrical systems. However, it is critical to confirm the voltage rating specified by the manufacturer and ensure compatibility with your clinic’s local power supply. Units intended for North America are commonly rated at 120V, while European and Asian markets often use 230V. Always verify the power adapter or internal power supply specifications and consider models with auto-switching capabilities for international use or multi-location practices. |
| 2. Are spare parts for dental scanner carts readily available, and how long are they supported? | Reputable manufacturers provide spare parts—including wheels, trays, power modules, and mounting brackets—for a minimum of 7–10 years post-discontinuation. In 2026, leading brands offer online spare parts portals for clinics and distributors, enabling quick procurement. We recommend selecting carts from OEMs with established service networks and regional distribution centers. Distributors should confirm spare parts availability and lead times before placing bulk orders to ensure long-term serviceability and minimize downtime for end-users. |
| 3. Does the dental scanner cart require professional installation, or can it be assembled in-house? | Most dental scanner carts in 2026 are designed for tool-free, plug-and-play assembly and do not require professional installation. Units typically arrive partially assembled, with clear instructions for final setup, including attaching the scanner mount, connecting power, and calibrating integrated components. However, clinics integrating carts with existing imaging systems or electronic health record (EHR) platforms may need technical support for seamless connectivity. Distributors should provide setup guides and remote assistance options to support end-clients during deployment. |
| 4. What is the standard warranty coverage for a dental scanner cart, and what does it include? | The standard warranty for dental scanner carts in 2026 is typically 2–3 years, covering defects in materials and workmanship under normal use. Coverage includes structural components (frame, shelves), electrical systems (integrated power strips, USB hubs), and mechanical parts (casters, height-adjustment mechanisms). Consumables (e.g., cable ties, non-slip pads) and damage from misuse or accidents are generally excluded. Extended warranty options up to 5 years are available through manufacturers and authorized distributors, often including on-site service and preventive maintenance. |
| 5. How do I verify compatibility with my existing intraoral scanner and clinic infrastructure? | Compatibility should be confirmed at three levels: physical, electrical, and digital. Physically, ensure the cart’s mounting system supports your scanner model (e.g., universal brackets, brand-specific holders). Electrically, verify sufficient power outlets and USB/USB-C ports for charging and data transfer. Digitally, confirm integration with clinic management software via Wi-Fi, Ethernet, or docking stations. Leading 2026 models support plug-and-play connectivity with major scanner brands (e.g., 3Shape, Align, Carestream). Distributors should maintain compatibility matrices and offer demo units for on-site testing. |
Note: Specifications and service policies may vary by manufacturer. Always consult technical datasheets and service agreements prior to purchase.
Need a Quote for Dental Scanner Cart?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


