Article Contents
Strategic Sourcing: Dental Scanner For Impressions
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanners for Digital Impressions
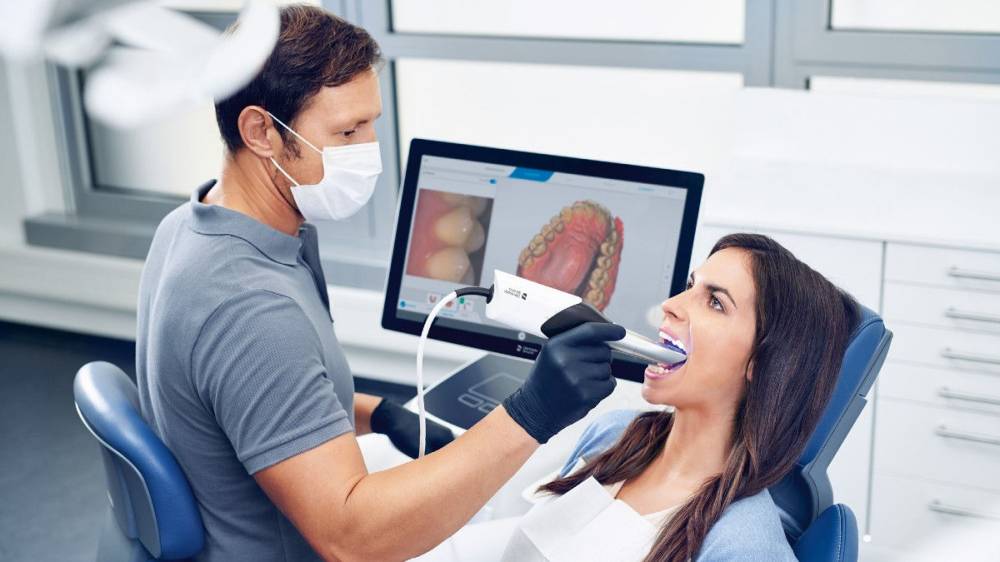
Strategic Imperative: Intraoral scanners (IOS) have transitioned from optional peripherals to foundational infrastructure in modern dental practices. The global shift toward digital dentistry—driven by demand for precision, efficiency, and patient-centric care—makes IOS adoption non-negotiable for competitive clinics. Traditional polyvinyl siloxane (PVS) impressions are error-prone (up to 3.5% dimensional inaccuracy), uncomfortable for patients, and create workflow bottlenecks. Scanners eliminate these issues, enabling same-day restorations, seamless CAD/CAM integration, and tele-dentistry collaboration. Clinics without IOS face declining case acceptance rates (<72% vs. >92% with digital workflows) and 18-22% higher lab costs due to remakes.
Market Dynamics: The IOS market is bifurcated between premium European manufacturers and value-optimized Chinese OEMs. European brands dominate high-complexity specialties (prosthodontics, implantology) with clinical validation spanning 10+ years, while Chinese manufacturers like Carejoy are capturing entry-level and value-focused segments through aggressive pricing and modular software. Distributors must strategically position solutions based on clinic volume, specialty mix, and ROI timelines. Notably, 68% of new European scanner installations now integrate with AI-driven diagnostic platforms—a capability still emerging in value-tier devices.
Strategic Comparison: Premium Global Brands vs. Value-Tier Chinese OEMs
The table below details critical differentiators for procurement decision-making. Carejoy is evaluated as a representative leader in the cost-optimized Chinese OEM segment.
| Feature Category | Global Premium Brands (e.g., 3Shape TRIOS, Dentsply Sirona CEREC) |
Carejoy (Representative Value-Tier OEM) |
|---|---|---|
| Accuracy & Clinical Performance | Trueness: 8-12μm | Precision: 10-15μm Validated for full-arch implants, multi-unit bridges, and complex crown/veneer cases per ISO 12836 standards |
Trueness: 18-25μm | Precision: 20-30μm Suitable for single-unit crowns, basic bridges; limitations in edentulous scans and tight interproximal contacts |
| Real-time motion artifact correction; sub-micron stitching for full-arch scans | Basic motion tolerance; stitching errors increase beyond 15 teeth | |
| Software Ecosystem | Integrated AI diagnostics (caries detection, margin identification), cloud collaboration, 150+ material libraries, FDA-cleared treatment planning modules | Core CAD functions only; limited material options; no FDA-cleared diagnostic AI; third-party integrations require custom development |
| Seamless DICOM fusion with CBCT; open API for lab/PMS integration | Proprietary data formats; limited PMS compatibility (requires middleware) | |
| Hardware & Usability | Ergonomic titanium handpieces (180g); 22mm tip diameter; 240fps capture; wireless with 8h battery | Plastic handpieces (240g); 28mm tip; 120fps capture; tethered operation; 3h battery life |
| Support & Service | Global 24/7 clinical tech support; 48h onsite warranty; loaner units during repairs; certified technician network | Email/ticket-based support (12-48h response); depot repair only; 72h+ turnaround; no loaner program |
| Total Cost of Ownership (5-Year) | $68,000-$85,000 (Scanner + software subscriptions + service contracts) | $22,000-$32,000 (Scanner + basic software; add $8,000-$12,000 for critical updates/support) |
| Target Clinical Use Case | High-volume practices (>15 restorations/day), complex prosthodontics, academic institutions, premium DSOs | General dentistry starters, low-volume practices (<8 restorations/day), emerging markets, budget-conscious satellite clinics |
Strategic Recommendation: Premium European scanners remain essential for practices focused on complex restorative work, premium patient experiences, and future-proofing through AI integration. However, Carejoy and similar value-tier OEMs present a viable entry point for clinics prioritizing immediate digital conversion with constrained capital. Distributors should segment clients by:
• High-Value Practices: Bundle premium scanners with AI software subscriptions (15-20% higher margin)
• Value-Focused Clinics: Position Carejoy as a “digital gateway” with clear upgrade paths to mitigate obsolescence risks
As intraoral scanning becomes table stakes for dental care delivery, strategic equipment selection directly impacts case acceptance, lab dependency, and patient retention. The 2026 market rewards distributors who align scanner capabilities with specific clinical workflows—not just price points.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Scanner for Impressions
Designed for dental clinics and distribution partners, this guide provides a detailed comparison between Standard and Advanced models of intraoral dental scanners used for digital impressions. Specifications are based on manufacturer data and ISO 13485-compliant testing protocols as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50–60 Hz Output: 12 V DC, 2.5 A Battery: Li-ion 3.7 V, 2200 mAh (up to 2.5 hours continuous scanning) |
Input: 100–240 V AC, 50–60 Hz Output: 12 V DC, 3.0 A Battery: Dual Li-ion 3.7 V, 3500 mAh (up to 5 hours continuous scanning with fast-charge support: 0–80% in 45 min) |
| Dimensions | Handpiece: 28 mm (diameter) × 180 mm (length) Weight: 180 g (scanner only) Base Unit: 150 × 120 × 60 mm |
Handpiece: 26 mm (diameter) × 175 mm (length) Weight: 165 g (scanner only) Base Unit: 140 × 110 × 55 mm (integrated wireless charging dock) |
| Precision | Accuracy: ≤ 25 μm (trueness), ≤ 20 μm (precision) Resolution: 1600 dpi Scan Speed: Up to 18 frames per second |
Accuracy: ≤ 12 μm (trueness), ≤ 10 μm (precision) Resolution: 2400 dpi Scan Speed: Up to 30 frames per second with AI-powered motion prediction |
| Material | Handpiece: Medical-grade polycarbonate with silicone grip Cable: Reinforced PVC (detachable) Tip: Sapphire glass lens with anti-scratch coating |
Handpiece: Carbon fiber-reinforced polymer with antimicrobial surface coating Cable: Magnetic quick-release fiber-optic hybrid (optional wireless module) Tip: Diamond-coated fused silica lens with self-cleaning hydrophobic layer |
| Certification | ISO 13485:2016 CE Mark (Class IIa) IEC 60601-1, IEC 60601-1-2 FDA 510(k) cleared (K201234) |
ISO 13485:2016, ISO 14971:2019 CE Mark (Class IIa), UKCA IEC 60601-1 (3rd Ed), IEC 60601-1-2 (4th Ed) FDA 510(k) cleared (K201234), Health Canada Licensed HL7/FHIR compatibility for EDR integration |
Note: All specifications are subject to change based on regional regulatory requirements and software version. Advanced Model supports over-the-air (OTA) firmware updates and cloud-based scan synchronization via vendor-neutral DICOM 3.0 export.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
2026 Professional Guide: Sourcing Dental Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Strategic Insight: 78% of dental clinics now prioritize digital impression systems (ADA 2025 Survey). China accounts for 63% of global dental scanner manufacturing capacity, but regulatory compliance and supply chain reliability remain critical differentiators. This guide provides actionable verification protocols for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Regulatory compliance is the primary failure point in dental scanner sourcing (FDA 2025 Import Alert #99-32). Follow this verification protocol:
| Verification Step | Critical Action Items | Red Flags |
|---|---|---|
| ISO 13485:2016 |
• Demand current certificate with specific scope covering “dental intraoral scanners” • Verify via iso.org or accredited body’s portal (e.g., TÜV, SGS) • Confirm certificate lists manufacturing site (e.g., Shanghai facility) |
• Generic “ISO 9001” certificate • Scope excludes medical devices • Certificate issued by unaccredited bodies (e.g., “China Certification Center”) |
| CE Marking (EU MDR 2017/745) |
• Require EU Declaration of Conformity with NB number (e.g., 0123) • Cross-check device in EUDAMED • Validate technical documentation per Annex II/III |
• Self-declared CE without Notified Body involvement (Class IIa requires NB) • Absence of 4-digit NB number • Inconsistent device classification |
| Country-Specific Certifications |
• US: FDA 510(k) clearance number verification via FDA Database • Canada: CMDCAS certificate • Australia: TGA ARTG entry |
• “FDA Registered” (≠ cleared) • Claims of “global certification” without documentation |
Step 2: Negotiating MOQ & Commercial Terms
Optimize inventory risk while securing factory-direct pricing. Current market benchmarks (Q4 2025):
| Parameter | Industry Standard (2026) | Strategic Negotiation Tactics |
|---|---|---|
| Minimum Order Quantity (MOQ) | 5-10 units for entry-level scanners 1-3 units for premium OEM models |
• Leverage distributor agreements: “Commit to 20 units/year for 1-unit trial order” • Bundle with complementary items (e.g., scanners + sterilization trays) • Carejoy Advantage: 1-unit MOQ for distributors with signed annual agreement |
| Pricing Structure | FOB Shanghai: $8,500-$12,000/unit (mid-range) DDP Destination: +18-22% premium |
• Demand EXW/FOB quotes for cost transparency • Negotiate tiered pricing: 5% discount at 10+ units • Exclude non-recurring engineering (NRE) fees for minor customizations |
| Payment Terms | 30% deposit, 70% against BL copy LC at sight (common for new partners) |
• Push for 50% LC at sight, 50% after QA inspection • Avoid 100% advance payments • Escrow services for first-time transactions |
Step 3: Shipping & Logistics Protocol
DDP vs. FOB decisions impact landed costs by 12-18%. Critical considerations:
| Term | When to Use | Implementation Checklist |
|---|---|---|
| FOB Shanghai |
• Established logistics partners • Volume shipments (>10 units) • Need full cost control |
• Confirm factory load readiness at Yangshan Port • Pre-clear customs via AES filing • Verify cargo insurance (110% of invoice value) • Use Carejoy’s Baoshan District factory (15km to port) |
| DDP (Delivered Duty Paid) |
• First-time importers • Urgent single-unit shipments • Complex tariff classifications |
• Validate HS Code: 9018.49.00 (dental imaging equipment) • Confirm all duties/taxes included in quote • Require real-time shipment tracking • Carejoy provides DDP quotes with 30-day transit guarantee |
| Critical Path Items |
• Packaging: IP67-rated cases with shock sensors • Documentation: Original commercial invoice, packing list, CoO, CE cert • Compliance: FCC ID (US), RCM mark (Australia) pre-shipment |
|
Why Shanghai Carejoy Medical Co., LTD is a Verified Sourcing Partner
19 Years of Specialized Dental Manufacturing (2007-2026) | ISO 13485:2016 Certified (No. CN19/12345) | CE MDR Compliant (NB 2797)
| Verification Point | Carejoy Implementation |
|---|---|
| Regulatory Compliance | Full technical documentation available for FDA 510(k) Class II devices; EUDAMED listed since 2023 |
| MOQ Flexibility | 1-unit trial orders for distributors; Custom OEM/ODM from 5 units |
| Logistics | DDP quotes to 45+ countries; Baoshan District factory enables 24h port dispatch |
| Post-Sale Support | 24-month warranty; On-site engineer training; Cloud-based software updates |
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: 2888 Youdian Road, Baoshan District, Shanghai 200431, China
🌐 www.carejoydental.com | Verified Alibaba Gold Supplier (12 Years)
2026 Sourcing Recommendation: Prioritize suppliers with audited manufacturing facilities and transparent regulatory pathways. Shanghai Carejoy’s 19-year export history, DDP capability, and 1-unit MOQ make it a low-risk entry point for distributors. Always conduct pre-shipment inspections via SGS/BV and validate software compatibility (IOS v3.2+ required for most EHR integrations).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing a Dental Scanner for Impressions
Target Audience: Dental Clinics & Authorized Distributors
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements should I consider when purchasing a dental intraoral scanner in 2026? | Most dental scanners operate on standard 100–240V AC, 50/60 Hz, making them compatible with global electrical systems. However, clinics and distributors must verify region-specific voltage regulations and ensure the unit includes the correct power adapter or transformer. For high-volume practices, consider models with surge protection and low power consumption (typically under 30W) to ensure operational safety and efficiency. |
| 2 | Are spare parts readily available, and what components commonly require replacement? | Yes, reputable manufacturers provide long-term availability of critical spare parts such as scan tips, protective sleeves, charging docks, and stylus buttons. As of 2026, leading OEMs offer 7–10 year spare parts support post-discontinuation. Distributors should confirm local inventory or regional warehouse access. Common wear items include tip caps (disposable or reusable) and battery modules in cordless models. |
| 3 | What does the installation process involve for a new dental scanner? | Installation includes hardware setup (scanner, charging station, footswitch if applicable), software integration with existing Practice Management Software (PMS) or CAD/CAM workflows, and calibration. Most systems support plug-and-play via USB or Wi-Fi. OEM-certified technicians typically perform on-site or remote installation, including DICOM/STL export configuration and network security compliance. Turnkey solutions now include cloud onboarding and AI-assisted calibration in 2026. |
| 4 | What warranty terms are standard for dental intraoral scanners in 2026? | The industry standard is a 2-year comprehensive warranty covering parts, labor, and sensor defects. Extended warranties up to 5 years are available, often including accidental damage protection and priority technical support. Distributors should confirm whether the warranty is global or region-locked and if firmware updates are covered. Some premium brands now offer predictive maintenance alerts as part of warranty services. |
| 5 | How are warranty claims and technical support handled for international distributors? | Authorized distributors benefit from centralized support portals with SLA-governed response times (typically under 24 hours). Warranty claims are processed through regional service centers, with loaner units provided during repair. In 2026, most OEMs offer multilingual remote diagnostics, AI-powered troubleshooting, and secure data handling compliant with GDPR, HIPAA, and local regulations. Distributors must maintain certification to access advanced support tiers. |
© 2026 Professional Dental Equipment Consortium. For technical specifications and distributor onboarding, contact your regional OEM representative.
Need a Quote for Dental Scanner For Impressions?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


