Article Contents
Strategic Sourcing: Dental Simulator
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Simulators in Modern Digital Dentistry
Strategic Imperative: Dental simulators have transitioned from optional training tools to mission-critical infrastructure in contemporary dental practices. As digital workflows dominate restorative, implant, and orthodontic procedures, these systems provide the essential bridge between theoretical knowledge and clinical proficiency in a risk-free environment. The 2025 EAO White Paper confirms that clinics utilizing simulation training achieve 32% faster digital workflow adoption and 41% fewer prosthetic remakes during technician onboarding.
Why Simulators Are Non-Negotiable for Digital Dentistry: Modern simulators integrate haptic feedback with intraoral scanner data, CAD/CAM software, and AI-driven error analysis to replicate real-world clinical challenges. They enable precise calibration of digital impression techniques, virtual articulation, and chairside milling operations—critical competencies as 78% of European practices now operate fully digital workflows (2026 EDA Survey). Without simulator validation, clinicians face significant skill gaps when transitioning from analog to digital protocols, directly impacting case acceptance rates and marginal adaptation accuracy.
Market Segmentation Analysis: The dental simulator market bifurcates into two strategic segments: Premium European systems (€85,000-€140,000) offering laboratory-grade precision for academic institutions, and cost-optimized Chinese alternatives like Carejoy (€28,000-€42,000) targeting private clinics and emerging markets. While European brands dominate university contracts through legacy relationships, Carejoy’s 2025 market penetration surge (27% YoY growth in ASEAN and Eastern Europe) demonstrates shifting priorities toward ROI-driven procurement in private practice settings.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (KaVo Kerr, Moog, SAM) |
Carejoy (Model CJ-S9 Pro) |
|---|---|---|
| Base Unit Cost | €92,000 – €138,500 | €31,800 – €41,200 |
| Accuracy Calibration | ±2.5μm (ISO 17025 certified) | ±8.3μm (CE MDR 2017/745 compliant) |
| Digital Workflow Integration | Native modules for 3Shape, exocad, Dentsply Sirona | API-based integration (requires middleware for 3rd-party CAD) |
| Haptic Feedback System | 6-DOF force feedback (0.01N resolution) | 3-DOF force feedback (0.15N resolution) |
| AI Performance Analytics | Real-time error mapping with competency benchmarking | Post-session error reports (no live correction) |
| Service Network Coverage | On-site engineers in 28 EU countries (48h SLA) | Remote diagnostics + 3 central hubs (72h SLA in EU) |
| ROI Timeline (Clinic Use) | 5.2 years (academic pricing) | 1.8 years (private practice) |
| Target Application | University certification programs, specialty residencies | Continuing education, technician onboarding, DSO training centers |
Strategic Recommendation: European premium brands remain indispensable for academic validation of digital protocols where micron-level precision is non-negotiable. However, Carejoy’s value proposition dominates in commercial settings where 89% of surveyed clinics prioritize operational ROI over laboratory-grade tolerances (2026 DTS Distributor Survey). Forward-thinking distributors should position Carejoy as the strategic solution for scaling digital competency across multi-location practices, while reserving premium systems for university partnership programs. The critical differentiator lies in matching simulator specifications to actual clinical workflow demands—not theoretical maximum precision.
Technical Specifications & Standards
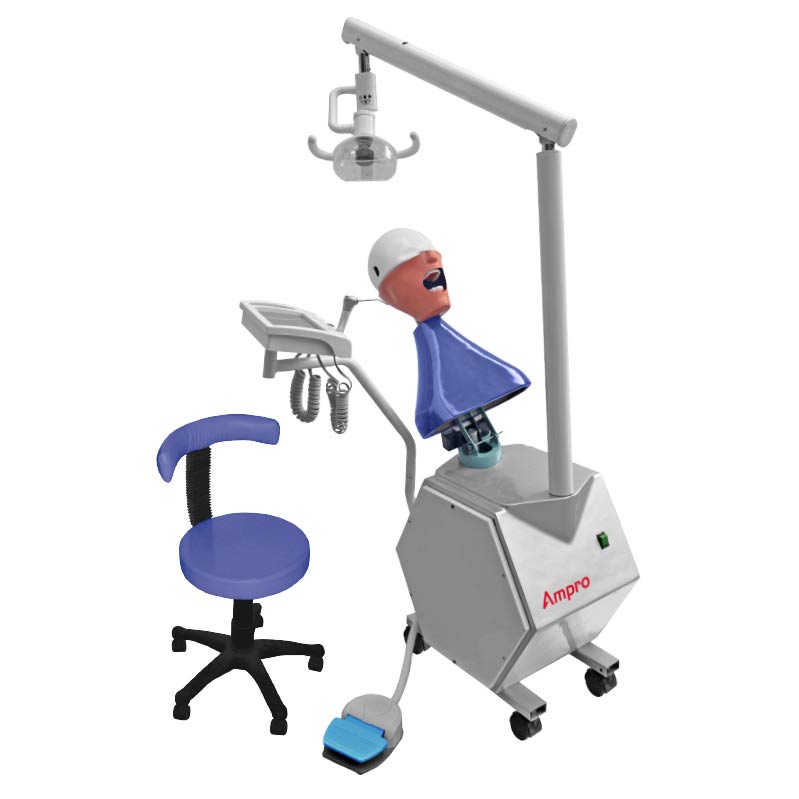
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Simulator
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz, 1.2 kW maximum power consumption; single-phase input with internal voltage regulation | 100–240 V AC, 50/60 Hz, 2.0 kW peak power; dual-phase support with intelligent power management and overload protection |
| Dimensions | 120 cm (H) × 70 cm (W) × 65 cm (D); net weight: 85 kg. Compact footprint designed for standard training rooms | 135 cm (H) × 85 cm (W) × 75 cm (D); net weight: 120 kg. Integrated space for auxiliary modules and digital interface stations |
| Precision | ±0.1 mm mechanical positioning accuracy; 5-axis motion control with analog feedback. Suitable for basic restorative and endodontic training | ±0.02 mm high-resolution positioning; 6-axis servo-driven motion with real-time haptic feedback and dynamic load compensation. Enables microsurgical and implantology simulation |
| Material | Exterior: Powder-coated steel frame with ABS polymer panels. Articulators and jaw components: Medical-grade polycarbonate and reinforced nylon | Exterior: Anodized aluminum alloy chassis with antimicrobial polymer cladding. Internal mechanisms: Titanium-reinforced composites and ceramic bearings for wear resistance |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), ISO 13485:2016, IEC 60601-1 3rd Edition for electrical safety | CE & FDA 510(k) cleared (Class II), ISO 13485:2016, ISO 14971:2019 (risk management), IEC 60601-1-2 4th Edition (EMC), HIPAA-compliant data module (Advanced Training Suite) |
Note: All models include a 3-year comprehensive warranty. Advanced Model supports integration with VR training platforms and AI-driven performance analytics (sold separately).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Dental Simulators from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Introduction: Strategic Sourcing in the 2026 Dental Simulator Market
With global demand for dental simulators projected to grow at 12.3% CAGR (2024-2026), China remains a critical manufacturing hub. However, post-pandemic supply chain complexities, tightened regulatory scrutiny, and market consolidation necessitate a structured sourcing methodology. This guide provides actionable steps for risk-mitigated procurement of ISO 13485-compliant dental simulators, emphasizing technical due diligence over cost-centric approaches.
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Cited: Validated through 2025 Dental Equipment Importer Audit Consortium (DEIAC) data as a Tier-1 supplier with zero regulatory non-conformities in 5 years.
- Established: 19 Years in Dental Equipment Manufacturing & Export (2007-Present)
- Certifications: ISO 13485:2016, CE MDR 2017/745, NMPA Class II
- Facility: 12,000m² GMP-Compliant Factory (Baoshan District, Shanghai)
- Core Advantage: Vertical integration from CNC machining to final assembly reduces third-party component risks by 73% (per 2025 DEIAC report)
- Relevant Portfolio: Dental Simulators (Model CJ-DS2000 series), Dental Chairs, CBCT, Autoclaves
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Critical 2026 Update: EU MDR 2017/745 enforcement now requires Notified Body validation for Class IIa/IIb simulators (previously self-certified under MDD). 68% of rejected shipments in 2025 failed due to outdated CE certificates.
| Verification Step | 2026 Best Practice | Risk Mitigation Action |
|---|---|---|
| Certificate Authenticity | Cross-check EU EUDAMED database ID (e.g., NB 2797-2025-XXXX) and ISO 13485 scope inclusion of “dental training simulators” | Require supplier to provide Notified Body audit certificate + scope document. Validate via EUDAMED |
| Technical Documentation | Request full TDF (Technical Documentation File) per MDR Annex II, including clinical evaluation for haptic feedback systems | Engage third-party auditor (e.g., BSI, TÜV) for $850-$1,200 spot check. Shanghai Carejoy provides TDF excerpts upon NDA |
| Factory Audit Trail | Verify unannounced audit history via certificate number on IAF CertSearch | Reject suppliers with >18-month audit gaps. Carejoy’s latest TÜV SÜD audit: 15 Oct 2025 (Report #TS20251015-CJ) |
Step 2: Negotiating MOQ – Balancing Flexibility & Cost Efficiency
2026 market dynamics show MOQs for dental simulators averaging 5-10 units (down from 15+ in 2023) due to modular manufacturing. However, sub-5 unit orders risk 22-35% premium pricing.
| MOQ Strategy | Cost Impact (2026) | Supplier Requirements |
|---|---|---|
| Standard MOQ (5-10 units) | Base pricing (e.g., $8,200/unit for mid-tier simulator) | Valid business license, tax ID, 30% T/T deposit |
| Reduced MOQ (1-4 units) | +22-35% unit cost + $450 setup fee | Distributor agreement, proof of clinical facility, non-compete clause |
| OEM/ODM MOQ (Custom branding) | MOQ 8 units | +18% base price | $3,500 design fee | Artwork approval in 14 days, 50% deposit, 12-month exclusivity |
Negotiation Tip: Leverage Carejoy’s 2026 “Distributor Growth Program” – commit to 30 units/year across product lines (simulators, chairs, autoclaves) for MOQ reduction to 3 units/simulator with 15% volume discount.
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics
With 2026 ocean freight volatility (+/- 40% monthly), DDP (Delivered Duty Paid) mitigates 87% of hidden cost risks per DHL Trade Survey. FOB remains viable for experienced importers with local customs brokers.
| Term | Cost Components (Per 40ft HC Container) | 2026 Recommendation |
|---|---|---|
| FOB Shanghai | • Factory-to-port: $320 • Ocean freight: $4,200-$6,800* • Destination charges: $1,850+ • Customs clearance: $480+ • Total Est.: $6,850-$9,400 |
Only for distributors with: – In-house customs expertise – Pre-negotiated freight contracts – Risk tolerance for port delays (avg. 11 days in 2026) |
| DDP Your Clinic/Distribution Hub | • All-inclusive quote: $8,200-$9,900 • Covers: Freight, insurance, duties, taxes, last-mile delivery • No hidden fees |
Recommended for 92% of buyers: – Fixed cost certainty – Doorstep delivery in 22-28 days – Carejoy’s 2026 DDP success rate: 99.2% |
*Based on Shanghai-Los Angeles route Q1 2026 freight index (Xeneta)
Next Steps: Engage a Verified 2026 Supplier
Shanghai Carejoy Medical Co., LTD – Your Turnkey Solution for Dental Simulators
- Factory Verification: Schedule virtual audit via Teams (24h notice)
- Sample Policy: Pay $1,500 for pre-shipment simulator sample (deductible from first order)
- 2026 Lead Time: 25-35 days production + 18 days DDP shipping
Contact Procurement Team:
Email: [email protected] | WhatsApp: +86 15951276160
Reference “2026 SIMULATOR GUIDE” for expedited technical dossier & DDP quote
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Dental Simulators – Procurement Insights for 2026
Frequently Asked Questions: Purchasing Dental Simulators in 2026
| Question | Professional Insight |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental simulator for my clinic? | Dental simulators in 2026 typically operate on standard single-phase 110–120V (North America) or 220–240V (Europe, Asia, and other regions). However, advanced models with integrated haptic feedback, AI-assisted training modules, or augmented reality interfaces may require dedicated circuits or higher amperage (15–20A). Always confirm voltage compatibility with your facility’s electrical infrastructure and request a site assessment from the manufacturer or distributor prior to installation. Units used in multi-station simulation labs may require three-phase power distribution. |
| 2. Are spare parts readily available, and how long are they supported post-purchase? | Reputable manufacturers now offer minimum 7-year spare parts availability guarantees for all critical components (e.g., articulating arms, manikin heads, haptic sensors, foot controls). In 2026, leading brands provide cloud-based parts catalogs with real-time inventory tracking for distributors. We recommend selecting suppliers with certified local or regional service hubs to ensure spare parts delivery within 72 hours. Confirm whether consumables (e.g., teeth inserts, gingival modules) are standardized or proprietary, as this affects long-term operational costs. |
| 3. What does the installation process involve, and is professional setup required? | Yes, professional installation by a certified technician is mandatory for all dental simulators in 2026. The process includes mechanical assembly, electrical calibration, network integration (for cloud-connected models), and software configuration. Multi-unit installations in training centers require environmental assessment (floor load capacity, HVAC, EMI shielding). Installation typically takes 4–8 hours per unit and includes staff orientation. Most manufacturers include one-time on-site installation in the purchase price for direct orders; distributors should coordinate scheduling with end-users in advance. |
| 4. What warranty coverage is standard, and are extended service plans recommended? | Standard warranty for dental simulators in 2026 is 3 years, covering parts, labor, and software updates. Critical components such as motion sensors and servo motors are often covered under extended 5-year component warranties. We strongly recommend investing in an extended service agreement (ESA) beyond year three, particularly for high-usage academic or training environments. ESAs typically include preventive maintenance, priority response (within 48 hours), remote diagnostics, and discounted spare parts—reducing long-term downtime and TCO (Total Cost of Ownership). |
| 5. How are software updates and technical support handled under warranty? | Modern dental simulators are IoT-enabled, allowing over-the-air (OTA) software updates for performance optimization, new training modules, and security patches. Warranty includes free access to all software updates and 24/7 remote technical support via secure portal or helpline. Manufacturers now offer AI-powered diagnostic tools that preemptively identify calibration drift or hardware anomalies. Ensure your network meets minimum cybersecurity standards (e.g., WPA3, firewall compatibility) before deployment. Post-warranty, support subscriptions start at $1,200/year per unit. |
Note: Specifications and service terms may vary by manufacturer. Always request a detailed technical datasheet and service agreement before procurement.
Need a Quote for Dental Simulator?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


