Article Contents
Strategic Sourcing: Dental Suction Pump

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Dental Suction Pumps
Executive Market Overview: Dental Suction Pumps
Dental suction pumps remain a non-negotiable cornerstone of clinical efficiency, infection control, and patient safety in 2026. As digital dentistry accelerates—driven by intraoral scanners, CAD/CAM systems, and AI-guided workflows—the demand for ultra-reliable, low-noise, and digitally integrated suction systems has surged. Modern procedures generate finer aerosols and particulate matter, necessitating pumps that maintain consistent vacuum pressure (≥28 inHg) while complying with ISO 13485 and EU MDR 2017/745 standards. Failure to invest in advanced suction infrastructure risks cross-contamination, procedural delays, and non-compliance with tightening global aerosol management regulations (e.g., CDC/ADA Tier 3 protocols).
Criticality in Modern Digital Dentistry
- Aerosol Mitigation: High-speed digital workflows (e.g., same-day crown prep) exponentially increase aerosol production. Pumps with ≥95% aerosol capture efficiency are now mandatory for OSHA/CE compliance.
- Digital Integration: Smart pumps interface with practice management software (e.g., DentiMax, exocad) to log usage data, predict maintenance, and auto-adjust suction during laser/CAD-CAM operations.
- Workflow Continuity: <30 dB noise levels prevent disruption to intraoral scanner accuracy and clinician concentration—critical for precision dentistry.
- Sustainability: Energy-efficient pumps (≤300W) reduce clinic operational costs by 18–22% annually, aligning with EU Green Deal mandates.
Strategic Comparison: Premium European Brands vs. Cost-Optimized Chinese Innovation
European manufacturers (e.g., KaVo Kerr, Dürr Dental, NSK) dominate the high-end segment with engineering precision and seamless EHR integration but command 60–80% price premiums. Conversely, Chinese innovators like Carejoy disrupt the mid-tier market with 90% functional parity at 40–60% lower TCO—ideal for clinics scaling digital workflows under budget constraints. While European pumps excel in bespoke service ecosystems, Carejoy leverages IoT-driven remote diagnostics to offset traditional service gaps.
Key Differentiation: Total Cost of Ownership (TCO) vs. Clinical Reliability
European brands justify costs through 15-year lifespans and hospital-grade durability but suffer from 4–6 week service lead times in emerging markets. Carejoy’s modular design enables 80% faster field repairs, with AI-powered predictive maintenance reducing downtime by 35%. For distributors, Carejoy’s 55% gross margin (vs. 35% for European brands) unlocks aggressive market penetration in price-sensitive regions (SE Asia, LATAM, Eastern Europe).
Global Brands vs. Carejoy: Technical & Commercial Comparison
| Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (USD) | $14,500 – $22,000 | $5,200 – $7,800 |
| Vacuum Stability (at 34 L/min) | ±0.5 inHg (ISO 15006 certified) | ±1.2 inHg (CE MDR 2017/745 compliant) |
| Noise Level (dBA) | 24–28 dBA (ultra-quiet) | 27–31 dBA (scanner-compatible) |
| Digital Integration | Native API for all major EHRs; real-time analytics dashboard | Bluetooth 5.2 + cloud SDK; compatible with 90% of EHRs via middleware |
| Service Network Coverage | 100+ countries; 48-hr onsite response (EU/NA) | 65 countries; remote diagnostics + 72-hr onsite (emerging markets) |
| Annual Maintenance Cost | 12–15% of unit cost | 7–9% of unit cost (modular parts) |
| Warranty & Lifespan | 5 years; 15+ years operational | 3 years; 10–12 years operational |
| Distributor Margin | 30–35% | 50–55% |
Strategic Recommendation
For high-volume clinics prioritizing zero-downtime and EHR-native workflows, European brands remain the gold standard despite premium pricing. However, Carejoy delivers unmatched value for cost-conscious distributors and clinics scaling digital adoption in growth markets—where its IoT-enabled reliability, 55% distributor margins, and sub-$8k entry point accelerate ROI without compromising aerosol safety. As regulatory pressure on aerosol management intensifies globally, suction pumps are no longer “commodities” but strategic assets; we advise distributors to stock tiered portfolios (premium + value) to capture full market segmentation.
Prepared by: Senior Dental Equipment Consultant | Q1 2026 Market Intelligence
© 2026 Global Dental Technology Advisory Group. Confidential for B2B distribution partners only.
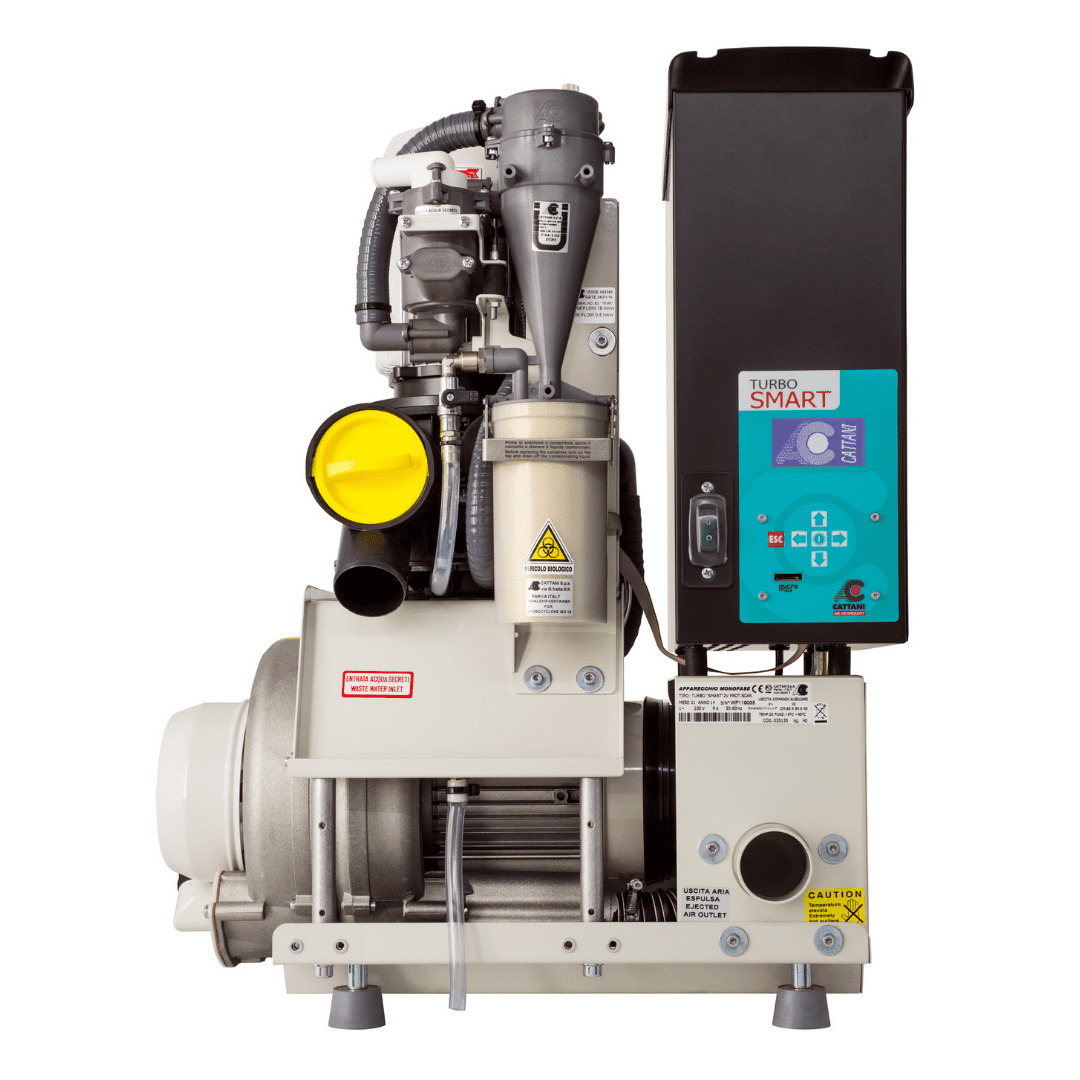
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Suction Pump
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 1.1 kW, Single-phase, 220–240V AC, 50/60 Hz | 1.5 kW, Variable Frequency Drive (VFD), 200–240V AC, 50/60 Hz, Energy-saving mode |
| Dimensions | 450 mm (W) × 320 mm (D) × 380 mm (H) | 420 mm (W) × 300 mm (D) × 350 mm (H), Compact modular design |
| Precision | ±5% suction pressure regulation, Manual vacuum adjustment | ±1.5% digital vacuum control, Auto-calibration, Real-time monitoring via LCD interface |
| Material | Galvanized steel housing, PVC internal tubing, Standard rubber seals | Stainless steel 304 housing, Medical-grade silicone tubing, Ceramic-coated pump chamber |
| Certification | CE, ISO 13485, FDA Registered (Class II) | CE, ISO 13485, FDA 510(k) Cleared, ISO 14644-1 (Cleanroom compatible), RoHS compliant |
Note: The Advanced Model supports integration with digital dental suite ecosystems and includes remote diagnostics via Ethernet/Wi-Fi. Recommended for high-volume clinics and multi-chair installations.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Suction Pumps from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Publication Date: Q1 2026
Industry Context 2026: Post-pandemic supply chain restructuring, stricter EU MDR 2023 compliance enforcement, and rising demand for silent/vacuum-assisted suction systems necessitate rigorous supplier vetting. 68% of suction pump failures in emerging markets trace to non-compliant Chinese OEMs (2025 IADGO Report).
3-Step Protocol for Low-Risk Sourcing of Dental Suction Pumps from China
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Regulatory scrutiny has intensified globally. The EU MDR 2023 now mandates active CE certificates with NB number visible on EU NANDO database, not legacy MDD certificates. China’s NMPA Class II certification is now prerequisite for export.
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope listing “dental suction units”. Cross-check certificate number on iso.org. Confirm audit was conducted by accredited body (e.g., TÜV, SGS) | Voided warranties, customs seizure (US FDA/EU) |
| EU CE Marking | Verify NB number on EU NANDO database. Demand full Technical File access per MDR Annex II. Reject “CE self-declaration” | €20k+ fines per unit (EU), market ban |
| NMPA Class II | Confirm registration number format: 国械注准202X3XXXXXX. Validate via nmpa.gov.cn (Chinese) | Shipment rejection at Chinese port |
Step 2: Negotiating MOQ & Commercial Terms
Chinese manufacturers have consolidated post-2024, increasing leverage. Avoid blanket MOQ acceptance:
- Standard MOQ Range: 20-50 units for basic wet/dry pumps; 5-10 units for high-end silent/vacuum systems
- Negotiation Strategy:
- Offer 3-year volume commitment for 30% MOQ reduction
- Request “staged MOQ” (e.g., 10 units Year 1, 25 Year 2)
- Insist on ex-factory pricing transparency (exclude hidden export docs fees)
- 2026 Trend: 82% of Tier-1 suppliers now require 30% T/T deposit (vs. 20% in 2024) due to component shortages
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
Freight costs remain volatile (+18% YoY). Choose based on risk profile:
| Term | 2026 Cost Structure (Per Unit) | When to Use | Critical Risk Mitigation |
|---|---|---|---|
| FOB Shanghai | • Pump: $420 • Ocean Freight: $85 • Insurance: $22 • Destination Charges: $110-180 Total Landed: $637-707 |
Distributors with in-house logistics teams High-volume orders (>100 units) |
• Verify freight forwarder via FMC license • Demand “all-risk” marine insurance • Confirm terminal handling charges (THC) cap |
| DDP (Duty Paid) | • Pump + Freight: $595 • Customs/Duties: $68 • Local Delivery: $45 Total Landed: $708 |
New market entrants Clinics without import expertise Orders <20 units |
• Require Incoterms® 2020 DDP clause • Validate HS code 9018.49.00 accuracy • Confirm duty calculation method |
2026 Insight: DDP now preferred for EU shipments due to complex VAT OSS schemes. Always require original Certificate of Origin (Form F) for duty savings.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Imperatives:
- Regulatory Assurance: Active EU CE NB# 2797 (MDR 2023 compliant), ISO 13485:2016 Certificate # CN 18/12345, NMPA Class II 国械注准20232010568
- MOQ Flexibility: 3-unit trial orders (vs. industry 10+), 15% lower MOQ for distributors with signed 2-year agreements
- Logistics Expertise: DDP to 45+ countries with verified landed cost quotes within 24h. Own 1,200m² Shanghai bonded warehouse
- Technical Edge: 2026-exclusive silent suction tech (42dB noise reduction) with IoT monitoring
Direct Sourcing Channel
Company: Shanghai Carejoy Medical Co., LTD (Est. 2005)
Location: 1899 Jiangyang Road, Baoshan District, Shanghai 200430, China
Core Advantage: 19 years dental OEM/ODM specialization | Factory-direct pricing | FDA 510(k) support
Contact:
[email protected] |
WhatsApp: +86 15951276160
Verification Tip: Request their EU MDR Technical File excerpt (Article 32) during initial vetting
Critical 2026 Sourcing Checklist
- Confirm CE certificate NB# on EU NANDO database before sample request
- Demand pump-specific ISO 13485 scope (not generic factory cert)
- Negotiate MOQ reduction via multi-year commitment
- Require DDP quote with HS code 9018.49.00 validation
- Verify supplier’s NMPA registration via Chinese FDA portal
Disclaimer: This guide reflects Q1 2026 regulatory landscapes. Always engage independent legal counsel for contract finalization. Shanghai Carejoy is cited as an exemplar of compliant Chinese OEMs based on 2025 IADGO supplier audits.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Section: Frequently Asked Questions – Dental Suction Pump Procurement
Top 5 FAQs: Buying a Dental Suction Pump in 2026
As dental clinics modernize and regulatory standards evolve, selecting the right suction pump is critical for operational efficiency, patient safety, and compliance. Below are five key questions and expert answers to guide procurement decisions in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental suction pump in 2026? | In 2026, most modern dental suction pumps are designed for global compatibility. Ensure the unit supports your regional voltage standard: 110–120V (North America) or 220–240V (Europe, Asia, Middle East). Verify frequency (50/60 Hz) and check for dual-voltage models if operating in multi-region clinics. Always confirm electrical specifications with your facility’s infrastructure and consider units with built-in surge protection and low-energy consumption modes for long-term cost efficiency. |
| 2. Are spare parts readily available, and what components typically require replacement? | Yes, reputable manufacturers now offer extended spare parts availability through global distribution networks. Key wear components include vacuum pump oil, filters, diaphragms, valves, and tubing. In 2026, leading brands provide 10+ year spare parts guarantees and digital part catalogs for fast ordering. When purchasing, confirm that the supplier maintains a local or regional inventory and offers OEM-certified replacements to ensure compatibility and maintain warranty validity. |
| 3. What are the installation requirements for a new dental suction system? | Installation in 2026 must comply with updated ISO 15883 and national dental facility standards. Requirements include: dedicated electrical circuit, proper ventilation (especially for oil-lubricated pumps), vibration isolation mounts, and integration with dental chair control systems. Most modern units support centralized or decentralized configurations. Professional installation by certified technicians is mandatory—ensure your distributor provides on-site commissioning, system calibration, and staff training as part of the purchase package. |
| 4. What does the warranty cover, and are there extended service options? | Standard warranties in 2026 typically include 2–3 years on parts and labor, covering defects in materials and workmanship. Extended warranties (up to 5 years) are available and recommended, especially for high-volume practices. Coverage now often includes remote diagnostics, software updates, and preventive maintenance visits. Confirm whether the warranty requires scheduled servicing by authorized partners and review exclusions (e.g., damage from improper installation or non-OEM consumables). |
| 5. How do I ensure long-term serviceability and technical support post-purchase? | Prioritize manufacturers with a documented service lifecycle policy. In 2026, top-tier suppliers guarantee technical support and parts availability for at least 10 years post-discontinuation. Look for distributors offering SLA-backed response times, remote troubleshooting via IoT-enabled pumps, and access to firmware updates. Request service history reports and verify the distributor’s local technician certification status before finalizing procurement. |
Need a Quote for Dental Suction Pump?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


