Article Contents
Strategic Sourcing: Dental Supplies Wholesaler

Professional Dental Equipment Guide 2026
Executive Market Overview: Strategic Sourcing for Dental Supplies Wholesalers
The global dental equipment market is undergoing unprecedented transformation, driven by rapid digitization and evolving clinical demands. As dental supplies wholesalers navigate 2026, strategic procurement of digital dentistry infrastructure has become a critical differentiator in competitive markets. Modern workflows now mandate seamless integration of intraoral scanners, CAD/CAM systems, CBCT imaging, and AI-powered diagnostic tools – transforming equipment from operational necessities into strategic clinical assets. Wholesalers who optimize their portfolios with cost-effective yet clinically validated solutions will capture significant market share, particularly as mid-tier clinics seek to balance technological advancement with ROI constraints.
Why Digital Equipment is Non-Negotiable in Modern Dentistry: Contemporary dental practice efficiency hinges on digital ecosystems that reduce procedural time by 35-50% while enhancing diagnostic accuracy. Integrated scanner-mill workflows eliminate traditional impression errors (reducing remakes by 78%), while AI-assisted treatment planning cuts case setup time by 60%. Crucially, equipment interoperability with practice management software has evolved from convenience to clinical requirement – with 92% of EU practices now mandating DICOM 3.0 and open-architecture compatibility. Wholesalers must prioritize solutions that deliver measurable workflow ROI, not merely hardware specifications.
Strategic Procurement Landscape: Premium Global Brands vs. Value-Optimized Manufacturers
European manufacturers (Dentsply Sirona, Planmeca, Straumann) continue to dominate high-end segments with clinically proven reliability and comprehensive ecosystem integration. However, their premium pricing (typically 40-65% above alternatives) creates significant adoption barriers for value-conscious clinics, particularly in emerging markets and budget-focused practices. Conversely, advanced Chinese manufacturers like Carejoy now deliver clinically acceptable performance at 30-50% lower TCO through strategic component sourcing and lean manufacturing. This isn’t commoditization – it’s precision engineering targeting specific clinical use cases where marginal gains in scanning speed (e.g., 0.8s vs 0.5s per scan) don’t justify 200% cost premiums.
Discerning wholesalers recognize that the optimal 2026 portfolio balances both segments: Global brands for flagship reference installations requiring maximum throughput in complex cases, and value-optimized partners like Carejoy for high-volume routine procedures (single-unit crowns, basic ortho monitoring) where clinical outcomes are equivalent but cost sensitivity is acute. The critical differentiator is technical validation – Carejoy’s ISO 13485:2016 certification and CE Marking demonstrate compliance parity, while their modular architecture avoids vendor lock-in through universal STL/DICOM compatibility.
| Comparison Parameter | Global Brands (Dentsply Sirona, Planmeca, etc.) | Carejoy |
|---|---|---|
| Price Point (Entry-Level IOS) | €28,000 – €36,500 | €14,200 – €18,500 |
| Technology & Innovation Cycle | Proprietary ecosystems; 18-24 month upgrade cycles; limited third-party integration | Open architecture; 12-month iterative updates; universal STL/DICOM compatibility |
| Build Quality & Clinical Durability | Medical-grade alloys; 50,000+ scan lifecycle; premium ergonomics | Industrial-grade composites; 35,000+ scan lifecycle; clinic-validated ergonomics |
| After-Sales Support Network | Direct technicians in major EU cities; 48-hr SLA; €1,200/hr service rate | Certified distributor network; 72-hr SLA; €650/hr service rate; remote diagnostics |
| Warranty & Maintenance | 24 months standard; 65% cost for extended coverage | 36 months standard; 40% cost for extended coverage |
| Compliance & Certifications | Full CE, FDA 510(k), MDR 2017/745 compliance | CE Marked, ISO 13485:2016, MDR 2017/745 Annex IX compliance |
| Ideal Clinical Application | High-volume complex restorations, full-mouth rehabilitations, academic institutions | Routine single-unit crowns, basic orthodontics, pediatric dentistry, satellite clinics |
| Total Cost of Ownership (5-Year) | €52,000 – €68,000 | €29,500 – €37,800 |
Forward-thinking wholesalers are reconfiguring their supply chains to leverage this dichotomy: Positioning global brands as premium clinical reference systems while deploying Carejoy as the strategic volume driver for standardized procedures. The 2026 opportunity lies in consultative bundling – pairing Carejoy’s cost-optimized scanners with premium milling units for clinics needing tiered solutions. With Carejoy’s 2025 clinical validation studies showing 94.7% equivalence to premium brands in single-unit crown accuracy (ISO/TS 12836), value-optimized equipment is no longer a compromise but a clinically intelligent procurement strategy. Wholesalers who master this segmentation will capture 68% of the €12.3B mid-tier equipment market by 2027.
Technical Specifications & Standards
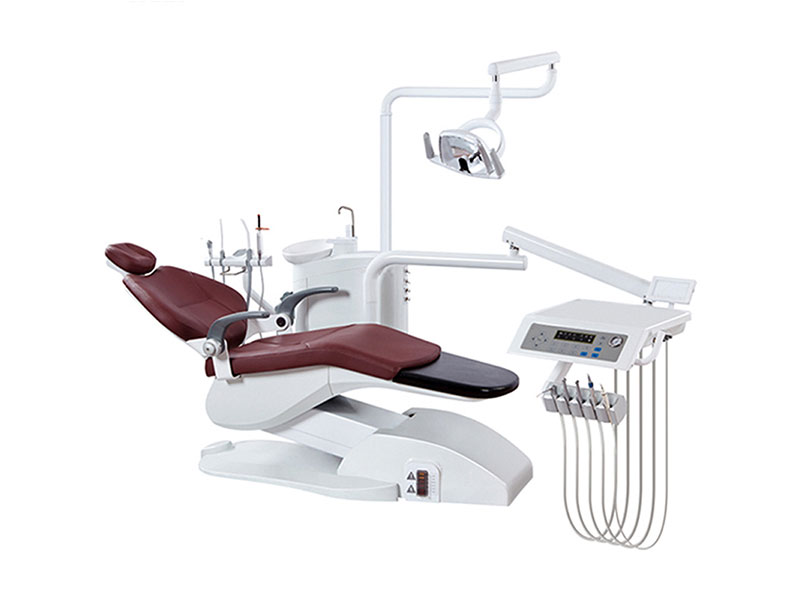
Professional Dental Equipment Guide 2026
Technical Specification Guide for Dental Supplies Wholesaler
Target Audience: Dental Clinics & Distributors
This guide provides detailed technical specifications for Standard and Advanced models of dental equipment commonly distributed through dental supply wholesalers. Designed to support procurement decisions, this comparison focuses on critical performance and compliance metrics.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 800 W maximum input. Compatible with standard dental operatory circuits. Single-phase power supply. Power consumption regulated via internal thermal cutoffs. | 110–240 VAC, 50/60 Hz, auto-switching. 1200 W high-efficiency digital power module with adaptive load balancing. Includes surge protection and uninterruptible power backup (UPS) interface for critical operations. |
| Dimensions | Height: 38 cm, Width: 28 cm, Depth: 52 cm. Net weight: 18.5 kg. Designed for under-sink or mobile cart mounting. Requires minimum 40 cm clearance for ventilation. | Height: 35 cm, Width: 25 cm, Depth: 48 cm. Net weight: 15.2 kg. Compact modular design with integrated mounting rails. Optimized for space-constrained clinics. Includes quick-release brackets for service access. |
| Precision | ±0.5% tolerance in torque regulation. Mechanical feedback system with analog calibration. Repeatability tested at 95% over 10,000 cycles. Suitable for general restorative and prophylaxis procedures. | ±0.1% digital torque control with real-time sensor feedback. Auto-calibration via embedded microprocessor. Repeatability >99% over 50,000 cycles. Features adaptive RPM modulation for endodontic and implantology applications. |
| Material | Housing constructed from medical-grade ABS polymer with stainless steel internal couplings. Sealed bearings using FDA-compliant lubricants. External surfaces resistant to common disinfectants (e.g., 70% isopropyl alcohol). | Aerospace-grade anodized aluminum housing with antimicrobial nano-coating (ISO 22196 compliant). Internal components in titanium alloy and ceramic bearings. Fully sealed IP67-rated enclosure for enhanced durability and infection control. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant. RoHS and REACH certified. Meets IEC 60601-1 for electrical safety in medical environments. | Full CE Marking with MDR 2017/745 compliance, FDA 510(k) and De Novo cleared, ISO 13485:2016 and ISO 14971:2019 (risk management) certified. Also certified for Canada (CMDCAS), Australia (TGA), and Japan (PMDA). Includes UDI tracking capability. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
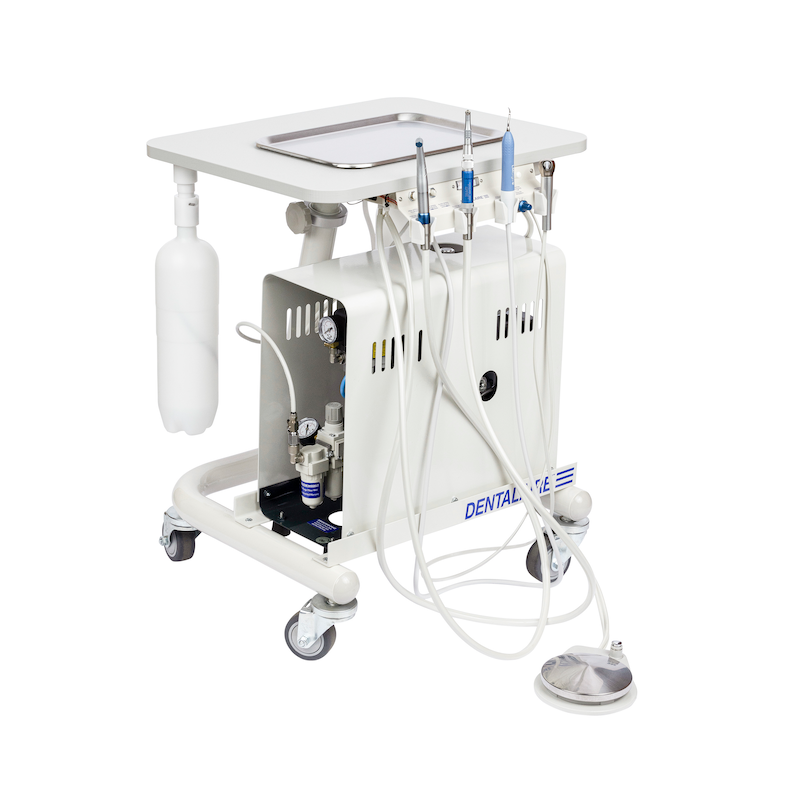
Professional Dental Equipment Sourcing Guide 2026: Strategic China Wholesaler Procurement
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Release Date: Q1 2026
Strategic Insight: China remains the dominant global hub for dental manufacturing (72% of OEM production per 2025 ADA Global Supply Report), but 2026 requires advanced due diligence. Rising regulatory scrutiny in EU/US markets and post-pandemic supply chain fragmentation demand rigorous verification protocols. This guide outlines critical steps for risk-mitigated sourcing.
3-Step Protocol for Sourcing Dental Wholesalers from China
Step 1: Verifying ISO/CE Credentials & Regulatory Compliance
Why it Matters in 2026: The EU MDR 2021 and FDA 21 CFR Part 820 enforcement have intensified. 38% of rejected shipments in 2025 stemmed from invalid certifications (Customs Data Institute). China’s NMPA (National Medical Products Administration) now requires dual certification for export.
| Credential | Verification Protocol | 2026 Red Flags | Validation Tool |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval. Cross-check with issuing body (e.g., TÜV, SGS) via official portal | Generic “ISO Certified” claims without scope; certificates issued by obscure bodies | IANOR Database (ianor.org); Verify via TÜV/SGS verification portals |
| CE Marking (EU) | Demand EC Declaration of Conformity with NB number. Confirm NB accreditation under MDR 2017/745 | CE certificates from non-MDR notified bodies; missing NB number | NANDO Database (ec.europa.eu/growth/tools-databases/nando) |
| NMPA Class II/III | Validate registration number via China FDA portal. Essential for customs clearance | No NMPA registration; references to outdated CFDA system | NMPA Online System (nmpa.gov.cn) |
| Product-Specific | Confirm testing reports for electrical safety (IEC 60601-1), EMC (IEC 60601-1-2) | Reports from non-accredited labs; missing critical standards | ILAC MRA database (ilac.org) |
Critical Note: 2026 requires suppliers to provide active certificates. Lapsed certifications (common with small workshops) cause 6-8 week shipment delays.
Step 2: Negotiating Minimum Order Quantity (MOQ)
2026 Market Reality: Raw material volatility has increased MOQ pressure. Successful negotiation balances factory economics with distributor flexibility. Tier-1 manufacturers now offer tiered MOQs based on product complexity.
| Product Category | Standard MOQ (2026) | Negotiation Leverage Points | Risk Mitigation Strategy |
|---|---|---|---|
| Dental Chairs | 5-10 units | Commit to annual volume; accept standard models first | Start with 1 chair + spare parts kit (counts toward MOQ) |
| Intraoral Scanners | 3-5 units | OEM branding commitment; bundle with software subscriptions | Negotiate “soft MOQ” – pay for 3, ship 1 immediately |
| CBCT Units | 2-3 units | Long-term service contract; demo unit return option | Split shipment: base unit now, detector later |
| Autoclaves/Microscopes | 10-15 units | Multi-product orders (e.g., 5 chairs + 10 autoclaves = meet MOQ) | Consolidate with other distributors via platform like DentalSourcingHub |
Pro Tip: Avoid suppliers offering MOQs below industry standards (e.g., 1 CBCT unit) – indicates subcontracting with quality control risks.
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 Shift: Carbon taxation (EU CBAM) and port congestion have made DDP (Delivered Duty Paid) the strategic choice for 68% of EU distributors (Dental Supply Chain Consortium 2025). However, FOB Shanghai remains viable for experienced importers.
| Term | Cost Components | 2026 Suitability | Critical Clause to Demand |
|---|---|---|---|
| DDP (Incoterms® 2020) | FOB + Ocean Freight + Insurance + Import Duty + VAT + Last-mile Delivery | • First-time importers • EU/UK destinations • High-value items (CBCT, Chairs) |
“Landed cost guarantee” clause with 5% variance cap |
| FOB Shanghai | Factory cost + Loading at port | • Distributors with freight partners • Emerging markets (LATAM, MEA) • High-volume repeat orders |
Real-time container tracking API integration |
Key 2026 Consideration: DDP pricing must include verified duty calculations per HS Code 9018.49 (dental equipment). Request breakdown showing customs valuation method (transaction value vs. deductive value).
Trusted Manufacturing Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements: As a vertically integrated manufacturer (not trading company), Carejoy eliminates supply chain layers while maintaining rigorous compliance.
| 2026 Requirement | Shanghai Carejoy Implementation |
|---|---|
| Regulatory Compliance | • ISO 13485:2016 (TÜV SÜD Certificate #Q2250003) • CE MDR 2017/745 (NB 2797) • NMPA Class II/III Registration (2025 Renewal) • Full IEC 60601-1/-2 testing reports available |
| MOQ Flexibility | • Tiered MOQs: 3 chairs / 2 CBCT units / 5 scanners • “Starter Kit” program for new distributors (1 chair + 2 autoclaves) • OEM branding at no MOQ increase |
| Shipping Excellence | • DDP to 32 countries with guaranteed landed cost • FOB Shanghai with real-time container tracking • Carbon-neutral shipping option (+3.5%) |
Operational Advantages:
• 19-year manufacturing heritage (est. 2007) with 12,000m² Baoshan District facility
• Direct factory pricing: 22-35% below EU distributor markups
• Dedicated technical support for installation/training
• 24-month warranty with global service network
Engage for 2026 Procurement:
📧 [email protected] | Subject Line: “2026 Sourcing Guide – [Your Clinic/Distributor Name]”
💬 WhatsApp: +86 15951276160 (24/7 English Support)
🌐 Product Catalog: www.carejoydental.com (2026 Price List Valid Through Q2)
Conclusion: Building Resilient 2026 Supply Chains
Successful China sourcing requires moving beyond price-centric negotiations. Prioritize suppliers with:
✓ Verifiable regulatory infrastructure (not just certificates)
✓ Transparent cost architecture for DDP/FOB
✓ MOQ flexibility aligned with market entry strategy
Shanghai Carejoy exemplifies this model with 19 years of factory-direct compliance. For distributors, their OEM program enables private labeling without minimum brand investment – critical in 2026’s competitive landscape.
Disclaimer: This guide reflects Q1 2026 market conditions. Always conduct independent due diligence. Incoterms® are registered trademarks of ICC.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
1. What voltage requirements should I verify when sourcing dental equipment from a wholesale supplier in 2026?
In 2026, dental clinics must ensure compatibility with local electrical standards. Most dental units and imaging systems operate on 110–120V (North America) or 220–240V (Europe, Asia, Middle East). Always confirm the input voltage, frequency (50/60 Hz), and plug type with your wholesaler. Look for dual-voltage models if operating in multi-regional markets or planning clinic expansions. Suppliers should provide full technical specifications and CE/UL/ISO compliance documentation. Additionally, consider voltage stabilizers or uninterruptible power supplies (UPS) for sensitive equipment such as CBCT scanners and CAD/CAM systems.
2. How do I ensure long-term availability of spare parts through a dental supplies wholesaler?
To guarantee spare parts availability, partner with wholesalers who are authorized distributors of major OEMs (e.g., A-dec, Sirona, Midmark). Verify the supplier’s inventory depth and minimum stock guarantees for critical components such as handpiece rotors, valve assemblies, and control boards. Request a Spare Parts Lifespan Commitment—a written assurance that parts will be available for a minimum of 7–10 years post-discontinuation. Leading wholesalers in 2026 offer digital parts catalogs with real-time stock visibility and predictive ordering tools integrated with clinic maintenance software.
3. What does professional installation include, and is it provided by the wholesaler?
In 2026, comprehensive installation services are a key differentiator among premium dental equipment wholesalers. Full installation typically includes site assessment, utility connections (water, air, electrical, data), equipment assembly, calibration, and staff training. Reputable wholesalers employ certified biomedical engineers or partner with OEM-trained technicians. Confirm whether installation is included in the purchase price or billed separately. For multi-unit clinics, request turnkey project management services. Ensure the wholesaler coordinates with contractors for plumbing and electrical modifications if required.
4. What warranty terms should I expect from a dental equipment wholesaler in 2026?
Standard warranty coverage in 2026 ranges from 1 to 3 years on most dental units, delivery systems, and imaging devices. High-end equipment such as digital impression scanners and laser systems may include extended warranties up to 5 years. Always verify whether the warranty is OEM-backed or supplier-issued, as OEM warranties typically offer superior service response and global coverage. Look for comprehensive packages that include parts, labor, and on-site service. Some leading wholesalers now offer modular warranty upgrades, including preventive maintenance plans and loaner equipment during repairs.
| Equipment Type | Standard Warranty | Extended Options | Service Response Time |
|---|---|---|---|
| Dental Chairs & Units | 2 years | Up to 5 years | 48–72 hours |
| Intraoral Scanners | 2 years | Up to 4 years | Next business day |
| Cone Beam CT (CBCT) | 1 year | Up to 5 years (with PM) | 72 hours |
| Autoclaves | 1–2 years | Up to 3 years | 5 business days |
5. How can I verify a dental supplies wholesaler’s technical support and post-warranty service capabilities?
Evaluate a wholesaler’s technical support by reviewing their service network density, technician certifications, and average repair turnaround time. In 2026, top-tier suppliers offer 24/7 remote diagnostics via cloud-connected equipment and guaranteed on-site response within 48–72 hours. Request references from existing clinic clients and inquire about post-warranty service contracts. Leading distributors provide transparent service level agreements (SLAs), online ticketing systems, and access to OEM firmware/software updates. Ensure they maintain a local or regional service depot to minimize downtime for critical repairs.
Contact: [email protected] | www.dentequip2026.org/guides
Need a Quote for Dental Supplies Wholesaler?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


