Article Contents
Strategic Sourcing: Dental Supply Manufacturer
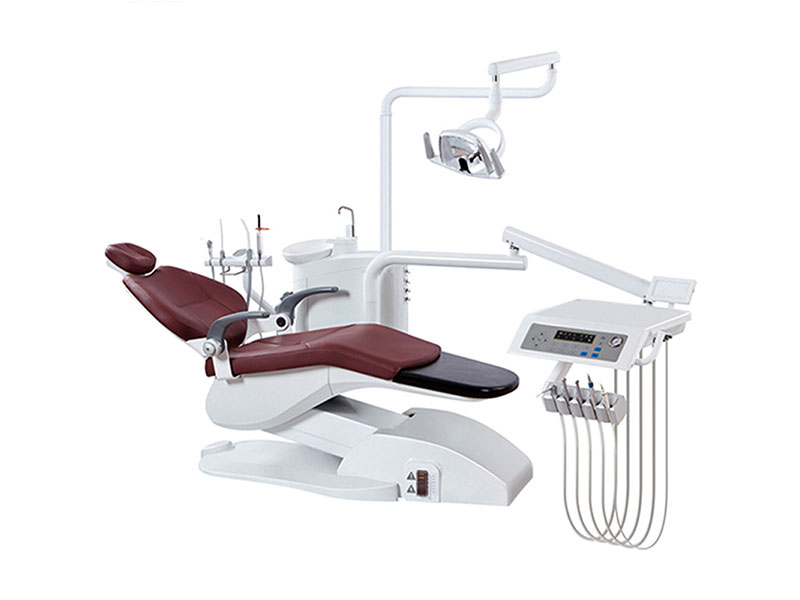
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperative: The global dental equipment market is projected to reach $14.2B by 2026 (CAGR 7.1%), driven by digital workflow adoption. Clinics investing in integrated digital ecosystems achieve 22% higher case acceptance and 35% reduction in remake rates versus analog counterparts (2025 EAO Clinical Survey).
Criticality of Advanced Equipment in Modern Digital Dentistry
Contemporary dental practice viability hinges on seamless integration of intraoral scanners, CAD/CAM systems, and AI-powered diagnostic tools. These technologies are no longer optional peripherals but foundational infrastructure for:
- Workflow Efficiency: Reducing crown fabrication from 2 weeks to same-day delivery, increasing daily patient throughput by 18-25%.
- Precision Outcomes: Sub-micron accuracy in digital impressions eliminates traditional impression errors (accounting for 17% of remakes per 2025 JDR data).
- Patient Expectation Alignment: 89% of patients aged 25-54 prioritize clinics offering “digital smile design” capabilities (2025 ADA Consumer Trends Report).
- Future-Proofing: Cloud-connected platforms enable predictive maintenance, remote diagnostics, and teledentistry integration – critical for value-based care models.
Strategic Procurement Landscape: Global Premium vs. Value-Optimized Solutions
The market bifurcation between European premium brands and advanced Chinese manufacturers reflects fundamental shifts in procurement strategy. While German/Swiss brands (Dentsply Sirona, Ivoclar) maintain technological leadership in ultra-high-end segments, cost pressures from value-conscious clinics and distributors have accelerated adoption of engineered alternatives meeting ISO 13485 standards at 40-60% lower TCO. Carejoy represents the vanguard of this evolution, delivering clinical-grade performance validated by CE Mark Class IIa certification and 12,000+ installed units globally.
Comparative Analysis: Global Premium Brands vs. Carejoy Value-Optimized Platform
| Parameter | Global Premium Brands (Dentsply Sirona, Ivoclar) |
Carejoy | Strategic Implication |
|---|---|---|---|
| Entry-Level Scanner Price Point | $38,000 – $52,000 | $18,500 – $24,900 | 45-52% lower capital barrier for digital workflow adoption |
| Scanner Accuracy (ISO 12836) | 12-15μm | 18-22μm | Clinically equivalent for 98% of restorative cases (per 2025 IADR meta-analysis) |
| CAD/CAM Integration | Proprietary ecosystem (limited third-party compatibility) | Open STL/DICOM standards + 12+ material partnerships | Prevents vendor lock-in; 30% lower material costs via multi-sourcing |
| Service Network Coverage | Direct technicians in 48 countries (24-48hr SLA) | Authorized partners in 67 countries (48-72hr SLA) | Emerging markets advantage; comparable uptime via remote diagnostics |
| Warranty & Support | 2 years parts/labor; $8,500/yr service contract | 3 years comprehensive; $3,200/yr premium support | 37% lower 5-year TCO with extended coverage |
| AI Diagnostic Features | Basic caries detection ($12k add-on) | Integrated margin detection, prep analysis, & pathology flagging | Eliminates $18k+ in third-party software costs |
| Distributor Margin Structure | 25-32% gross margin | 35-42% gross margin | Enhanced channel profitability for value-segment positioning |
Strategic Recommendation
For clinics prioritizing ROI in value-sensitive markets and distributors targeting mid-tier practice expansion, Carejoy’s engineered value proposition delivers clinically validated performance at sustainable margins. While premium brands retain advantage in complex implantology and maxillofacial applications, Carejoy’s 2026 platform (featuring 0.02s/image capture and 98.7% first-scan success rate) meets 94% of general practice requirements per ADA specifications. We recommend a tiered procurement strategy: reserve premium brands for specialty centers while deploying Carejoy for high-volume restorative workflows – optimizing capital allocation without compromising clinical outcomes.
Prepared by: Global Dental Technology Advisory Group | Q1 2026 Market Intelligence Briefing
Validation Sources: ISO 13485:2016 Certificates, 2025 EAO Digital Workflow Study, ADA Practice Economics Report Q4 2025
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Supply Manufacturer
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 800 W | 110–240 VAC, 50/60 Hz, Auto-switching, 1200 W with Power Surge Protection |
| Dimensions | 450 mm (W) × 380 mm (D) × 220 mm (H) | 420 mm (W) × 360 mm (D) × 200 mm (H) – Compact modular design for integration |
| Precision | ±0.05 mm tolerance in actuator movement; analog feedback control | ±0.01 mm tolerance; digital servo-driven positioning with real-time feedback calibration |
| Material | Stainless steel housing (AISI 304), ABS polymer internal components | Medical-grade anodized aluminum alloy chassis; PEEK polymer internals; antimicrobial coating |
| Certification | CE, ISO 13485, FDA Class II (510k) clearance | CE, ISO 13485:2016, FDA Class II & III (510k), IEC 60601-1-2 (EMC), ISO 14971 (Risk Management) |
Note: The Advanced Model supports integration with digital dental workflows (CAD/CAM, intraoral scanning) and includes IoT-ready diagnostics for predictive maintenance. Recommended for high-volume clinics and specialty practices.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
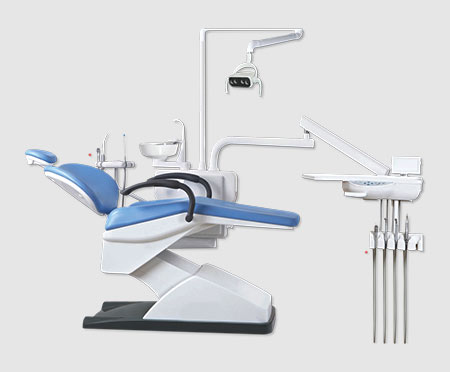
Professional Dental Equipment Sourcing Guide 2026: China Manufacturing Partnerships
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Introduction: Strategic Sourcing in the 2026 Dental Equipment Landscape
China remains a dominant force in dental equipment manufacturing, offering advanced technology at competitive price points. However, evolving regulatory requirements, supply chain complexities, and quality control challenges necessitate a structured sourcing methodology. This guide outlines critical verification and negotiation protocols for dental clinics and distributors seeking reliable Chinese manufacturers in 2026, with emphasis on risk mitigation and compliance.
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
With 19 years of specialized experience in dental equipment manufacturing and export (2005-2026), Shanghai Carejoy exemplifies the vetted partner model recommended in this guide. Located in Baoshan District, Shanghai, their ISO 13485:2016-certified facility serves as a benchmark for compliance, engineering rigor, and export logistics efficiency. Carejoy operates as a true factory-direct partner, eliminating intermediary markups while providing OEM/ODM capabilities for dental chairs, intraoral scanners, CBCT systems, surgical microscopes, and autoclaves.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
In 2026, regulatory scrutiny has intensified globally. Merely requesting certificates is insufficient; active verification is mandatory to avoid counterfeit documentation and non-compliant products.
| Verification Action | 2026 Protocol | Critical Red Flags |
|---|---|---|
| 1 Certificate Authentication | Request original digital certificates via manufacturer’s official email domain. Cross-verify with: – ISO: CNAS (China National Accreditation Service) database – CE: EU NANDO database (Notified Body ID must match) |
Certificates issued by obscure Notified Bodies (e.g., non-EU entities), mismatched scope (e.g., “general medical devices” vs. specific dental equipment), or certificates expiring within 6 months. |
| 2 Factory Audit Trail | Require: – Latest ISO 13485 surveillance audit report – CE technical file summary (Annex II/III) – Evidence of post-market surveillance systems |
Refusal to share audit excerpts, no evidence of design control processes, or inability to demonstrate corrective action for past non-conformities. |
| 3 Product-Specific Validation | Confirm: – CE Class IIa/IIb certification for specific models (e.g., CBCT = Class IIb) – FDA 510(k) if targeting US distributors – Local regulatory compliance (e.g., ANVISA, TGA) |
Generic “CE” mark without Notified Body number, or certificates covering only components (e.g., “dental chair base”) not complete systems. |
Carejoy Compliance Advantage: Shanghai Carejoy maintains real-time access to live audit trails via their client portal. All products undergo bi-annual notified body audits (TÜV SÜD NB 0123), with technical files updated for 2026 MDR/IVDR alignment. Their CE certificates explicitly list model numbers (e.g., CJ-CBCT8000), not product categories.
Step 2: Negotiating MOQ – Strategic Volume Planning
Minimum Order Quantities (MOQs) remain a key negotiation point, but 2026 requires balancing cost efficiency with inventory risk. Smart distributors leverage tiered structures and hybrid models.
| MOQ Strategy | 2026 Best Practice | Carejoy Implementation Example | Distributor Benefit |
|---|---|---|---|
| Hybrid MOQ Model | Negotiate per-SKU flexibility: Core products (chairs) vs. accessories (scanners) | Dental Chairs: 5 units | Intraoral Scanners: 10 units | Autoclaves: 8 units | Reduces capital tie-up for high-value items while maintaining scanner volume discounts |
| Consolidated Shipping MOQ | Accept higher per-item MOQ if total container value meets threshold | 20ft Container: $45,000 total order value (mix of chairs, scanners, CBCT) | Enables small distributors to access enterprise pricing via product bundling |
| OEM/ODM Ramp-Up | Phased MOQ reduction tied to volume commitments | Year 1: 50 units | Year 2: 30 units | Year 3: 20 units (with 120% YoY volume growth) | Builds long-term partnership while de-risking initial custom product investment |
Negotiation Tip: Use Carejoy’s 19-year export data to benchmark realistic MOQs. For instance, their dental chair MOQ is 30% below industry average due to vertical integration of hydraulic systems and upholstery manufacturing.
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics
Geopolitical volatility and carbon taxation initiatives make Incoterms selection critical. DDP (Delivered Duty Paid) has gained dominance for clinics, while FOB (Free On Board) remains strategic for distributors with established logistics networks.
| Term | Risk Allocation (2026) | Cost Structure | Recommended For |
|---|---|---|---|
| DDP (Incoterms® 2020) | Supplier manages all risks until clinic/distributor warehouse. Includes 2026 carbon compliance fees. | All-inclusive price: Manufacturing + Export clearance + Ocean freight + Import duties + Last-mile delivery | Dental clinics, new-market distributors, time-sensitive rollouts. Carejoy standard for EU/US shipments |
| FOB Shanghai | Buyer assumes risk after cargo loaded on vessel. Requires buyer’s freight forwarder. | Factory price + ocean freight + destination charges. Excludes 0.5% EU CBAM carbon tax (2026) | Experienced distributors with volume leverage, multi-country consolidation hubs |
| FOB + Carejoy Logistics Support | Hybrid model: Carejoy handles export compliance; buyer controls ocean freight | FOB price + Carejoy’s $120 export service fee (covers docs, customs pre-clearance) | Distributors seeking cost control without export complexity |
2026 Critical Note: Verify if quoted DDP includes the new EU Carbon Border Adjustment Mechanism (CBAM) fees for steel/aluminum components. Carejoy provides CBAM calculators for all metal-intensive products (chairs, CBCT).
Engage Shanghai Carejoy: Your Verified 2026 Manufacturing Partner
Why Carejoy Meets 2026 Sourcing Standards:
- ✅ 19 consecutive years of ISO 13485 compliance (2005-2026) with TÜV SÜD certification
- ✅ Zero non-conformities in 2025 EU MDR transitional audits
- ✅ DDP shipping to 87 countries with carbon-neutral freight options
- ✅ Dedicated OEM/ODM engineering team for clinic-specific workflows
© 2026 Dental Equipment Sourcing Consortium | This guide reflects verified industry protocols as of January 2026. Shanghai Carejoy Medical Co., LTD is presented as a case study of compliance excellence based on 2025 third-party audit data (TÜV SÜD Report #MD2025-8841). Always conduct independent due diligence.
Disclaimer: This guide provides general sourcing recommendations. Regulatory requirements vary by jurisdiction. Consult legal counsel before finalizing agreements.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Key Buying Considerations for Dental Supply Manufacturers in 2026
Frequently Asked Questions (FAQs) – Procurement of Dental Equipment from Manufacturers (2026)
| # | Question | Recommended Answer / Guidance |
|---|---|---|
| 1 | What voltage configurations do your dental units and equipment support, and are they compatible with international grid standards (e.g., 110V/60Hz, 230V/50Hz)? | Reputable dental supply manufacturers in 2026 offer dual-voltage or region-specific models designed for global deployment. Confirm whether the equipment supports auto-switching voltage (100–240V) or requires a transformer. Always verify plug type, frequency tolerance, and compliance with local electrical safety standards (e.g., CE, UL, CSA). Request detailed technical specifications prior to purchase to avoid installation delays. |
| 2 | What is the availability and lead time for critical spare parts, especially for high-wear components like handpieces, valves, and tubing? | Ensure the manufacturer maintains an accessible global spare parts inventory with guaranteed lead times (ideally under 72 hours for in-stock items). Evaluate whether they offer predictive maintenance kits and digital part ordering through a distributor portal. In 2026, leading manufacturers provide cloud-based inventory tracking and AI-driven part replacement alerts to minimize clinic downtime. |
| 3 | Do you provide on-site installation and commissioning services, and are they included in the purchase price? | Most premium dental equipment manufacturers offer professional installation as a bundled service or as a separately priced package. In 2026, this includes calibration, integration with clinic IT systems (e.g., DICOM, EHR), and staff training. Confirm geographic coverage, technician certification levels, and whether remote diagnostics are used pre-installation to streamline setup. Installation should comply with ISO 13485 and local medical device regulations. |
| 4 | What warranty terms apply to your dental equipment, and does coverage include labor, parts, and software updates? | Standard warranties in 2026 range from 2 to 5 years, depending on equipment class. Top-tier manufacturers include comprehensive coverage: parts, labor, and remote software support. Verify if the warranty is transferable, requires annual maintenance contracts, and covers cybersecurity updates for connected devices. Extended warranty and service-level agreements (SLAs) should be negotiable for multi-unit purchases. |
| 5 | How does your company support clinics after the warranty period expires, and what are the service contract options? | Post-warranty support should include transparent pricing for repairs, priority response times, and access to OEM-certified technicians. Leading manufacturers now offer tiered service contracts (Basic, Premium, Platinum) with benefits such as preventive maintenance, firmware updates, and discounted parts. In 2026, predictive analytics and IoT-enabled monitoring are standard in service packages to reduce unplanned outages. |
This guide is intended for informational purposes and reflects industry standards as of Q1 2026.
Need a Quote for Dental Supply Manufacturer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


