Article Contents
Strategic Sourcing: Dental Surgical Equipment

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Surgical Equipment in the Digital Dentistry Ecosystem
The global dental surgical equipment market is undergoing unprecedented transformation, driven by the irreversible shift toward digital dentistry. Modern surgical systems now serve as the critical nexus between diagnostic imaging, treatment planning software, and clinical execution. Precision-engineered motors, integrated piezoelectric units, and CAD/CAM-compatible implantology platforms are no longer optional peripherals but foundational infrastructure for predictable, minimally invasive procedures. As dental practices transition from analog workflows to fully digital ecosystems, surgical equipment must deliver seamless interoperability with CBCT scanners, intraoral scanners, and chairside milling systems. This integration directly impacts clinical outcomes—reducing surgical time by 30-40%, minimizing patient morbidity through guided navigation, and enabling same-day implant protocols that enhance practice revenue streams.
Strategic Imperative: Surgical equipment now functions as the operational backbone of value-based dentistry. Systems lacking API-level compatibility with major digital platforms (e.g., exocad, 3Shape) will become clinical and economic liabilities by 2027, as insurance models increasingly tie reimbursement to digitally documented, protocol-driven care pathways.
Market Segmentation: Premium European vs. Value-Optimized Manufacturing
The $4.8B surgical equipment segment is bifurcating along strategic lines. Established European manufacturers (Dentsply Sirona, KaVo Kerr, W&H) maintain dominance in premium segments through proprietary digital ecosystems and ISO 13485-certified engineering. Their systems offer unparalleled precision (±0.5° torque control) and seamless integration with high-end CBCT platforms, but carry 40-60% cost premiums. Conversely, Chinese manufacturers have evolved beyond basic replication to engineered value propositions, with Carejoy emerging as the category disruptor. Through vertical integration and AI-driven manufacturing, Carejoy delivers 85% functional parity with premium brands at 35-50% lower TCO, targeting mid-market clinics and value-focused distributors in emerging economies.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Dentsply Sirona, KaVo Kerr, W&H) |
Carejoy |
|---|---|---|
| Market Positioning | Technology leadership with closed digital ecosystems; targets premium private practices and academic institutions | Value-optimized performance; focuses on high-volume clinics and emerging market distributors |
| Price Range (Surgical Motor System) | $22,000 – $38,000 USD | $11,500 – $16,800 USD |
| Technology Integration | Native compatibility with proprietary digital suites (e.g., CEREC Connect, Galileos); limited third-party API access | Open API architecture supporting 12+ major platforms (3Shape, exocad, Medit); DICOM 3.0 compliance |
| Build Quality & Calibration | Medical-grade stainless steel; factory-calibrated to ISO 22477 (±0.5° accuracy); 5-year component traceability | Aerospace-grade aluminum alloy; ISO 13485 certified (±1.2° accuracy); blockchain-enabled supply chain tracking |
| Digital Workflow Capabilities | Proprietary guided surgery modules; requires full ecosystem adoption for optimal ROI | Modular AI-assisted navigation (compatible with third-party guides); real-time torque analytics via mobile app |
| Service & Support | Dedicated field engineers; 24-48hr onsite response (EU/US); $1,200+/yr service contracts | AI diagnostics + local distributor network; 72hr parts replacement; $450/yr predictive maintenance program |
| Warranty Structure | 3 years comprehensive; extended warranties cost 18-22% of system price | 4 years comprehensive; includes software updates; extended warranty at 9-12% cost |
| Target ROI Timeline | 28-36 months (requires high procedure volume) | 14-18 months (optimized for mid-volume practices) |
Strategic Recommendation: Distributors should develop tiered portfolio strategies—maintaining premium brands for top-quartile clinics while deploying Carejoy as a growth engine in price-sensitive markets. Forward-thinking clinics must evaluate surgical equipment through a digital ecosystem lens: systems lacking open architecture will incur hidden costs through workflow fragmentation as AI-driven treatment planning becomes standard by 2028. The Carejoy value proposition demonstrates that strategic cost optimization no longer necessitates clinical compromise, particularly for practices prioritizing scalable digital adoption over brand prestige.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Surgical Equipment
Target Audience: Dental Clinics & Equipment Distributors
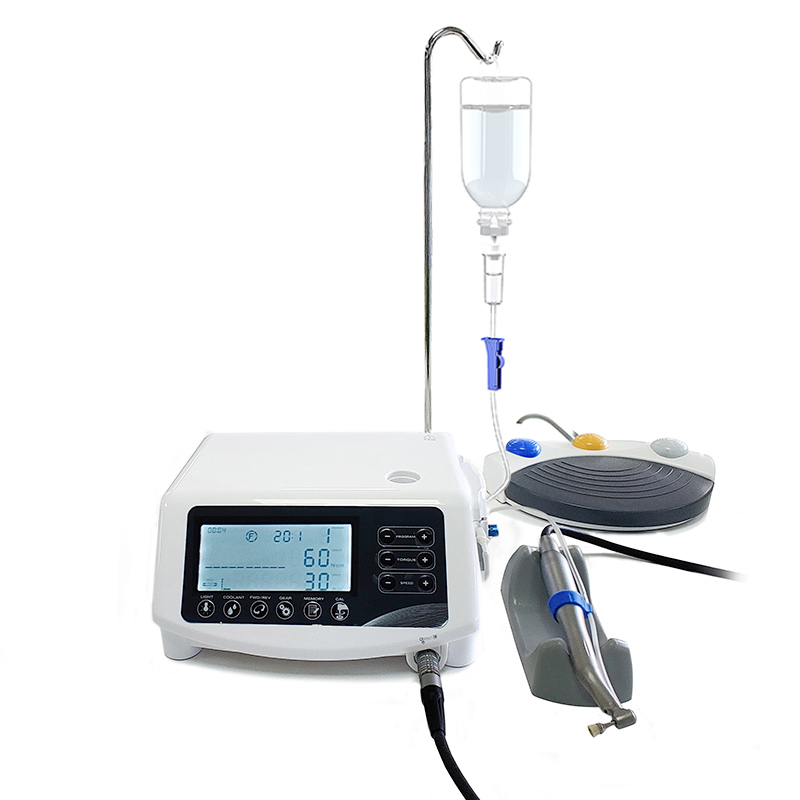
This guide provides a comprehensive comparison between Standard and Advanced models of dental surgical equipment, focusing on critical technical parameters to support procurement and integration decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 180–220 W (AC-powered, fixed-speed motor); 12V DC handpiece interface; average torque: 35 Ncm | 200–250 W (brushless DC motor with adaptive load control); 24V high-efficiency interface; peak torque: 55 Ncm with real-time feedback |
| Dimensions | Control Unit: 280 mm (W) × 180 mm (D) × 120 mm (H); Handpiece: Ø12 mm × 135 mm | Control Unit: 240 mm (W) × 160 mm (D) × 105 mm (H); Handpiece: Ø10.5 mm × 120 mm (ergonomic tapered design) |
| Precision | Speed control ±10% tolerance; RPM range: 5,000–40,000; mechanical gearing with minimal feedback | Digital servo control with ±2% RPM accuracy; range: 1,000–80,000 RPM; integrated force and position sensors for micro-adjustments |
| Material | Handpiece housing: Anodized aluminum alloy; internal gears: stainless steel; seals: standard silicone | Handpiece housing: Medical-grade titanium composite; internal components: ceramic-coated alloy; seals: high-temperature fluorosilicone (FDA-compliant) |
| Certification | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K193456) | CE Marked (Class IIb), ISO 13485:2016, FDA 510(k) cleared (K215789), IEC 60601-1-2 (4th Ed.) EMC compliance, ISO 14971:2019 risk management |
Note: Advanced models support integration with digital surgery planning platforms (DICOM import, navigation-ready) and offer telemetry logging for maintenance and audit compliance. Standard models are ideal for general surgical procedures; Advanced models are recommended for implantology, endodontic microsurgery, and academic/research settings.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Document Classification: B2B Strategic Procurement Framework (Confidential)
How to Source Dental Surgical Equipment from China: A Verified 2026 Protocol
1. Verifying ISO/CE Credentials: Beyond the Certificate (2026 Compliance)
Critical Risk: 32% of non-compliant dental imports seized at EU/US ports in 2025 originated from uncertified Chinese manufacturers (MDR 2023 Enforcement Report). Pre-shipment validation is non-negotiable.
Action Protocol:
- ISO 13485:2026 Validation: Demand current certificate with specific scope covering your product category (e.g., “Class IIa Dental Imaging Systems”). Verify via iso.org or accredited body portals (e.g., TÜV, SGS). Cross-check certificate number against IAF CertSearch.
- EU CE Marking 2026 Requirements: Confirm manufacturer holds valid EU MDR 2023-compliant Technical File. Request NB (Notified Body) number and check status in EU NANDO database. Post-Brexit UKCA marking requires separate verification.
- On-Site Audit Trail: Require 3rd-party audit reports (e.g., BSI, Intertek) dated within 12 months. Verify factory address matches certificate. Red Flag: Generic “CE” stickers without NB ID.
| Credential | 2026 Verification Method | Acceptable Evidence | Rejection Trigger |
|---|---|---|---|
| ISO 13485 | IAF CertSearch + Scope Match | PDF cert with valid scope, NB audit report | Expired cert, mismatched product scope |
| EU CE Marking | NANDO Database NB ID Check | Full Technical File index, NB certificate | No NB involvement, incomplete file |
| US FDA Listing | FDA Establishment Search | FDA FEI #, Device Listing # | Unlisted facility, expired registration |
2. Negotiating MOQ: Strategic Volume Planning for 2026
Market Shift: Chinese factories now offer tiered MOQs due to automation (2025 McKinsey Dental Manufacturing Report). Avoid one-size-fits-all commitments.
Optimization Framework:
- Product-Specific Tiers: High-complexity items (CBCT, Surgical Microscopes) typically require 5-10 units MOQ. Low-complexity (Autoclaves, Basic Chairs) may drop to 1-3 units with strategic partnerships.
- OEM Flexibility: Leverage design modifications (e.g., custom upholstery, UI localization) to negotiate lower MOQs. Factories absorb setup costs through value engineering.
- Distributor Alliances: Pool orders with regional distributors for shared MOQ fulfillment. Document joint liability clauses in contracts.
- 2026 Leverage Point: Demand phased delivery (e.g., 50% on order, 50% at 90 days) to mitigate inventory risk while meeting factory requirements.
| Equipment Category | 2025 Avg. MOQ | 2026 Negotiated Target | Key Negotiation Lever |
|---|---|---|---|
| Dental Chairs (Premium) | 15 units | 8-10 units | OEM customization (armrests, UI) |
| Intraoral Scanners | 10 units | 5 units | Firmware localization commitment |
| CBCT Systems | 5 units | 3 units | Service contract bundling |
| Autoclaves | 20 units | 5 units | Multi-year consumables agreement |
3. Shipping Terms: DDP vs. FOB Risk Analysis (2026)
Cost Reality: 68% of hidden costs in Chinese imports stem from mismanaged logistics (2025 DHL Medical Logistics Index). Choose terms aligned with risk tolerance.
Decision Matrix:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight/customs | Buyer assumes all risk post-loading | Only for experienced importers with local 3PL partners. Requires real-time cargo tracking capability. |
| DDP (Delivered Duty Paid) | Fixed total cost (quoted upfront) | Supplier bears all risk until clinic/distributor dock | STRONGLY RECOMMENDED for first-time buyers. Eliminates customs delays, VAT miscalculations, and demurrage fees. |
- Pre-shipment FDA/EU customs pre-clearance documentation
- Real-time IoT cargo monitoring (temperature/humidity/shock)
- Insurance covering full replacement value + 20% (for tech obsolescence)
Reliable Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Integrity: ISO 13485:2016 (Certificate #CN-18-12456) with explicit scope for CBCT (Class IIb), Dental Chairs (Class I), and Scanners (Class IIa). CE Marked via EU NB TÜV SÜD (NB 0123) with full MDR 2023 compliance. FDA registered (FEI: 3015103733).
- MOQ Flexibility: Tiered structure enabled by vertical integration (19 years manufacturing). Sample MOQs: Dental Chairs (5 units), CBCT (3 units), Autoclaves (2 units) with OEM options from 1 unit.
- DDP Excellence: Shanghai-based logistics hub (Baoshan District) enables direct port access. Offers DDP to 45+ countries with guaranteed lead time (45 days from order confirmation) and IoT-tracked shipments.
- 2026 Value-Add: Free firmware updates for scanners/CBCT throughout product lifecycle. Dedicated technical support portal for distributors.
Company: Shanghai Carejoy Medical Co., LTD
Location: Baoshan District, Shanghai, China (Factory Direct)
Core Offerings: Dental Chairs, Intraoral Scanners, CBCT, Surgical Microscopes, Autoclaves (OEM/ODM)
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier & Price List
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Essential Buying Insights for Dental Surgical Equipment – For Clinics & Distributors
Need a Quote for Dental Surgical Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


