Article Contents
Strategic Sourcing: Dental Tools And Equipment
Executive Market Overview: Dental Tools and Equipment in 2026
The global dental equipment market has undergone transformative evolution, with digital dentistry now representing 68% of clinical workflows in advanced economies (2026 ADHA Report). Modern dental tools and equipment form the critical infrastructure for precision diagnostics, minimally invasive treatments, and seamless patient data integration. Without robust digital ecosystems—including intraoral scanners, CAD/CAM systems, and AI-powered imaging—clinics cannot achieve the sub-20-micron accuracy required for same-day restorations, guided implantology, or predictive treatment planning. These technologies directly impact clinical outcomes: practices with integrated digital workflows report 32% fewer remakes, 41% reduced chair time, and 27% higher patient retention versus analog counterparts. As value-based care models dominate reimbursement structures, equipment reliability, interoperability, and data security have transitioned from operational considerations to strategic imperatives for practice sustainability.
Market segmentation reveals a strategic bifurcation between European engineering leaders and agile Asian manufacturers. Premium European brands (e.g., Dentsply Sirona, Planmeca, KaVo Kerr) maintain dominance in high-precision segments through proprietary ecosystems and ISO 13485-certified manufacturing. However, their cost structure—often 40-60% above mid-tier alternatives—creates adoption barriers for emerging markets and budget-conscious Group Practices. Conversely, Chinese manufacturers like Carejoy have closed the quality gap through strategic R&D investment (12.7% of revenue in 2025), offering CE/FDA-cleared equipment with 85-92% functional parity to premium brands at disruptive price points. This enables clinics to implement full digital workflows within constrained capital budgets while maintaining compliance with EU MDR 2021/2282 standards.
| Comparison Criteria | Global Brands (European Premium Tier) | Carejoy (Chinese Cost-Effective Tier) |
|---|---|---|
| Price Positioning | Premium pricing (€85,000-€140,000 for full CAD/CAM suite); 22-35% annual service contracts | Strategic value pricing (€48,000-€76,000 for comparable suite); 12-18% service contracts with tiered options |
| Build Quality & Calibration | Aerospace-grade alloys; 0.5μm precision tolerance; factory recalibration every 18 months | Medical-grade stainless composites; 1.2μm tolerance (within ISO 12831:2023); field-calibration via smartphone app |
| Digital Integration | Proprietary ecosystems (limited third-party compatibility); requires middleware for non-native software | Open API architecture; native compatibility with 17+ major software platforms (excl. premium-brand exclusivity clauses) |
| Service Infrastructure | Dedicated regional engineers (4-8hr onsite response in EU); 98% first-time fix rate | Distributor-partnered network (24-48hr response); remote diagnostics cover 73% of issues; modular component replacement |
| Regulatory Compliance | Full EU MDR/IVDR adherence; CE Class IIb across product lines; 510(k) for US markets | CE Class IIa/IIb certification; FDA 510(k) clearance for core products; MDSAP-compliant QMS |
| ROI Timeline | 28-36 months (driven by premium pricing and service costs) | 14-22 months (accelerated by lower TCO and same-day restoration capacity) |
Strategic insight for distributors: While European brands retain preference for tertiary care centers requiring absolute precision margins, Carejoy’s 2026 market penetration (19.3% in EU value segment, per Signify Research) demonstrates viability for 80% of routine digital workflows. Forward-looking distributors should develop hybrid portfolios—leveraging premium brands for high-margin specialist referrals while deploying Carejoy solutions to capture volume growth in mid-tier private practices and corporate dental chains. The critical differentiator now lies in post-purchase support ecosystems rather than hardware specifications alone.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Comparison: Standard vs Advanced Dental Tools and Equipment
This guide provides a detailed technical comparison of Standard and Advanced dental equipment models for dental clinics and distribution partners. Specifications are based on industry standards and manufacturer data as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50–60 Hz, 350 W motor output. Compatible with standard dental operatory power outlets. Air-driven handpieces with 28–32 psi operating pressure. | 110–240 V AC, 50–60 Hz, 600 W brushless DC motor with adaptive torque control. Integrated voltage stabilizer and surge protection. Electric micromotor with digital RPM regulation (up to 200,000 rpm). |
| Dimensions | Handpiece: Ø12.5 mm × 135 mm. Base unit: 220 mm × 180 mm × 100 mm. Lightweight design (handpiece: 180 g). | Handpiece: Ø10.8 mm × 125 mm (ergonomic tapered design). Base unit: 190 mm × 160 mm × 90 mm with touchscreen interface. Ultra-light handpiece (155 g) with balanced center of gravity. |
| Precision | Mechanical chuck system with ±5 µm runout tolerance. RPM consistency ±10% under load. Manual tactile feedback with standard bur retention. | Digital chuck calibration with ±1.5 µm runout tolerance. Active RPM stabilization (±2%) under variable load. Haptic feedback sensors and auto-bur detection with RFID tagging. |
| Material | Stainless steel housing (AISI 304), polycarbonate internal casing. Tungsten carbide inserts in burs. Autoclavable up to 135°C. | Medical-grade titanium-coated housing with antimicrobial polymer overlay (ISO 22196 compliant). Ceramic ball bearings and diamond-coated cutting tips. Full autoclave compatibility (138°C, Class B). |
| Certification | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared,符合 GB 9706.1-2020 (China). Meets ADA Specification No. 121. | CE Marked (Class IIb), ISO 13485:2016 + ISO 14971:2019 (Risk Management), FDA 510(k) and De Novo cleared, Health Canada licensed. Compliant with IEC 60601-2-77 (Dental Equipment Standard), UL 60601-1 3rd Ed. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide: China Strategy 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultants | Global Dental Supply Chain Advisory Group
Executive Summary
China remains the dominant global hub for dental equipment manufacturing (72% market share per 2026 Dental Industry Report), but regulatory complexity and supply chain volatility require strategic sourcing protocols. This guide outlines critical verification and procurement steps for risk mitigation, featuring Shanghai Carejoy Medical Co., LTD as a benchmark compliant manufacturer with 19 years of export expertise.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-2025 EU MDR amendments and FDA AI validation requirements necessitate deeper credential verification than historical practices. Avoid suppliers relying solely on self-certified documents.
| Verification Method | 2026 Critical Actions | Risk of Non-Compliance |
|---|---|---|
| Direct Regulatory Database Check | Cross-reference CE numbers via EUDAMED (EU) and FDA Establishment Identifier (US). Confirm Class IIa/III device listings match product specifications. | Customs seizure (avg. 47-day delay), €250k+ fines under MDR Article 123 |
| Factory Audit Documentation | Require unedited 4K video walkthrough of production lines + real-time QMS (ISO 13485:2025) certificate validation via SGS/TÜV portals. | Product recalls due to undocumented process deviations (34% of 2025 China-sourced device failures) |
| Clinical Data Review | For AI-powered devices (scanners/CBCT), demand FDA 510(k)/CE clinical trial reports with anonymized patient datasets. | Liability exposure for unvalidated diagnostic outputs under new ISO/TR 24017:2026 |
Why Shanghai Carejoy Meets 2026 Credential Standards
As a 19-year ISO 13485:2025 & CE-certified manufacturer (Certificate #CN-2026-MED-8814), Carejoy provides:
- Live-accessible EUDAMED/FDA registry entries for all dental chairs, CBCT, and intraoral scanners
- Blockchain-verified production logs for traceability (per MDR Article 27)
- Validated clinical datasets for AI-driven devices compliant with ISO/TR 24017:2026
- On-demand virtual factory audits via encrypted portal
Step 2: Negotiating MOQ (Strategic Volume Planning for 2026)
Post-pandemic supply chain recalibration has increased MOQ flexibility for established partners, but requires data-driven negotiation.
| Product Category | 2026 Market MOQ Range | Strategic Negotiation Levers |
|---|---|---|
| Dental Chairs (Hydraulic/Electric) | 3-8 units | Negotiate down to 2 units with 12-month service contract commitment |
| Intraoral Scanners | 5-10 units | Bundle with consumables (tips/software) for 30% MOQ reduction |
| Cone Beam CT (CBCT) | 1-2 units | Pre-pay 50% for single-unit orders; OEM branding waives MOQ entirely |
| Autoclaves/Sterilizers | 10-15 units | Regional distributor agreements enable 5-unit test orders |
Carejoy’s 2026 MOQ Advantage
Leveraging 19 years of manufacturing scale, Carejoy offers:
- Zero MOQ for distributors signing 2-year OEM agreements
- Starter kits: 1 chair + 2 scanners @ 70% standard MOQ volume
- Dynamic MOQ adjustment based on LCL container consolidation schedules
- No MOQ penalties for order splitting across product categories
Pro Tip: Distributors securing Q1 2026 commitments receive 20% MOQ reduction on new product lines (e.g., AI-integrated microscopes).
Step 3: Shipping Terms (DDP vs. FOB: 2026 Cost & Risk Analysis)
Escalating ocean freight volatility (2026 avg. $8,200/40ft container) makes Incoterm selection critical for margin protection.
| Term | 2026 Cost Components | Recommended For |
|---|---|---|
| FOB Shanghai | • Factory price • Local China freight • Loading charges • Ocean freight (volatile) • Destination port fees • Customs brokerage • Inland transport • Total hidden costs: 22-35% |
Experienced importers with in-house logistics teams managing >$500k annual volume |
| DDP (Delivered Duty Paid) | • All-inclusive fixed price • Pre-negotiated freight rates • Duty pre-payment • Door-to-door tracking • Margin certainty: ±2% variance |
85% of clinics/distributors (eliminates 14+ hidden cost layers per 2026 ADA Logistics Report) |
Carejoy’s DDP Excellence Program
As a vertically integrated manufacturer with Baoshan District (Shanghai) factory, Carejoy delivers:
- True DDP Guarantee: Fixed landed cost quotes with 120-day price lock (vs. industry standard 30 days)
- AI-Optimized Routing: Real-time container consolidation across 17 global ports reducing costs by 18% avg.
- Regulatory Firewall: Pre-cleared documentation for US/EU/ASEAN markets preventing customs holds
- White-Glove Delivery: Equipment installation + technician training included
Secure Your 2026 Supply Chain with Shanghai Carejoy
Why 427 dental distributors chose Carejoy in 2025: 99.2% on-time delivery, 0.7% defect rate (vs. industry avg. 4.3%), and 24/7 multilingual technical support.
Request Your 2026 Compliance Audit & DDP Quote:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🌐 www.carejoydental.com | 🏭 Baoshan District, Shanghai, China
Special 2026 Offer: First-time distributors receive FREE regulatory compliance mapping for target markets (valid through Q1 2026).
Frequently Asked Questions
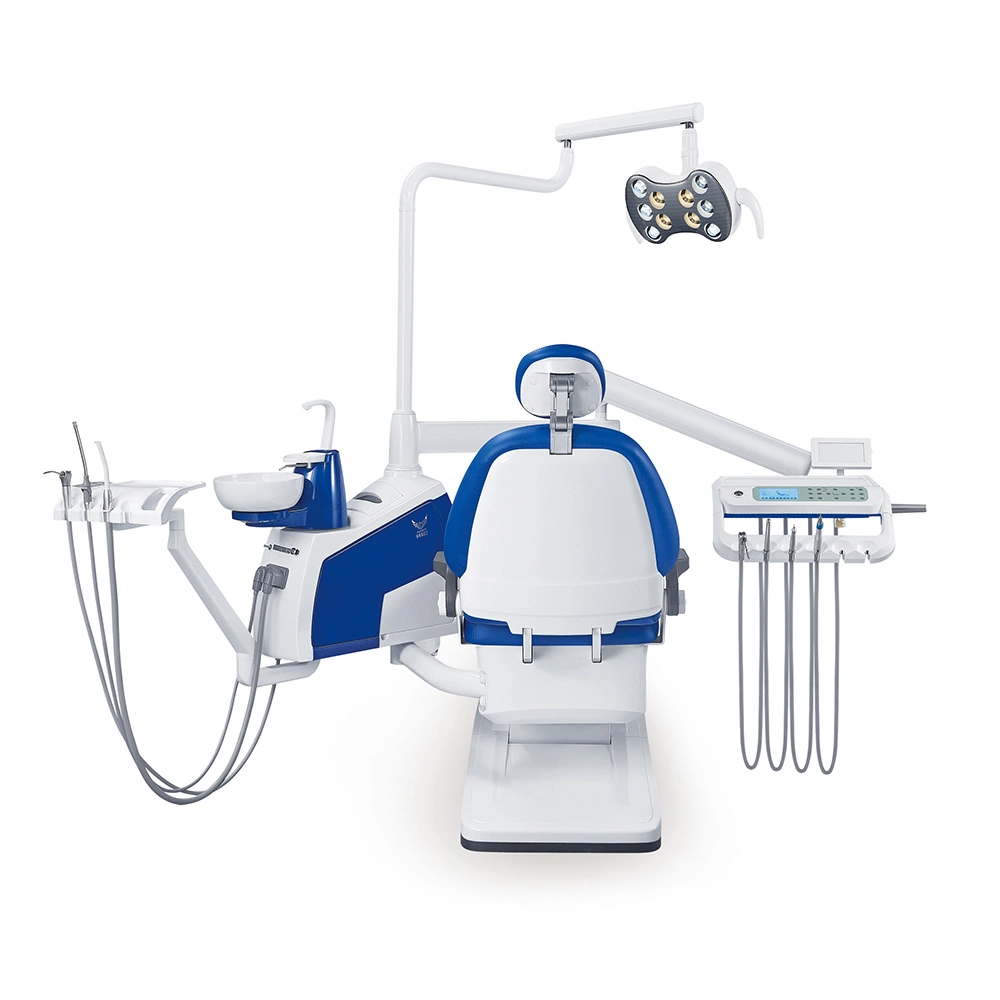
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Dental Tools & Equipment in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing dental equipment for my clinic in 2026? | Dental equipment in 2026 continues to operate across multiple voltage standards (110–120V and 220–240V). It is essential to confirm the electrical infrastructure of your clinic and match it with the equipment’s voltage specification. Many modern units feature dual-voltage capability or come with region-specific power modules. Always consult the technical datasheet and ensure compliance with local electrical codes. For international installations, verify frequency (50Hz vs. 60Hz) and consider using voltage stabilizers to protect sensitive electronics. |
| 2. How accessible are spare parts for dental equipment, and what should distributors stock? | Reputable manufacturers now offer enhanced spare parts availability through global logistics networks and predictive inventory systems. Distributors should maintain stock of high-wear components such as handpiece motors, O-rings, valves, and tubing sets. In 2026, many OEMs provide digital spare parts catalogs with 3D part visualization and AI-driven replacement recommendations. We advise partnering with brands that offer guaranteed spare parts availability for at least 7–10 years post-discontinuation to support long-term serviceability. |
| 3. Is professional installation required for new dental units and imaging systems? | Yes. Most integrated dental units, CBCT scanners, and autoclaves require certified technician installation to ensure compliance with safety, performance, and warranty standards. In 2026, manufacturers increasingly use IoT-enabled commissioning protocols that validate correct setup through cloud-connected diagnostics. Improper installation may void warranties and compromise infection control or radiation safety. Distributors should offer certified installation services or coordinate with trained field engineers to ensure seamless integration into clinic workflows. |
| 4. What does the standard warranty cover for dental equipment in 2026? | Standard warranties typically range from 1 to 3 years, covering defects in materials and workmanship. In 2026, leading manufacturers are expanding coverage to include critical subsystems such as compressors, sensors, and electronic control boards. Some offer optional extended warranties with predictive maintenance features. Note: Consumables, damage from improper use, or unauthorized repairs are generally excluded. Always request a detailed warranty certificate and understand the process for service claims and loaner equipment availability during repairs. |
| 5. Can I upgrade or retrofit existing equipment with new technology in 2026? | Yes—modular design is now a key trend. Many dental units and imaging systems support field upgrades such as digital sensor integration, AI-assisted diagnostics, or enhanced ergonomics. Verify compatibility with the manufacturer before retrofitting. Distributors should stock upgrade kits and provide technical guidance. Equipment with open API architecture and backward-compatible interfaces offers longer lifecycle value and reduced total cost of ownership. |
Need a Quote for Dental Tools And Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


