Article Contents
Strategic Sourcing: Dental Unit
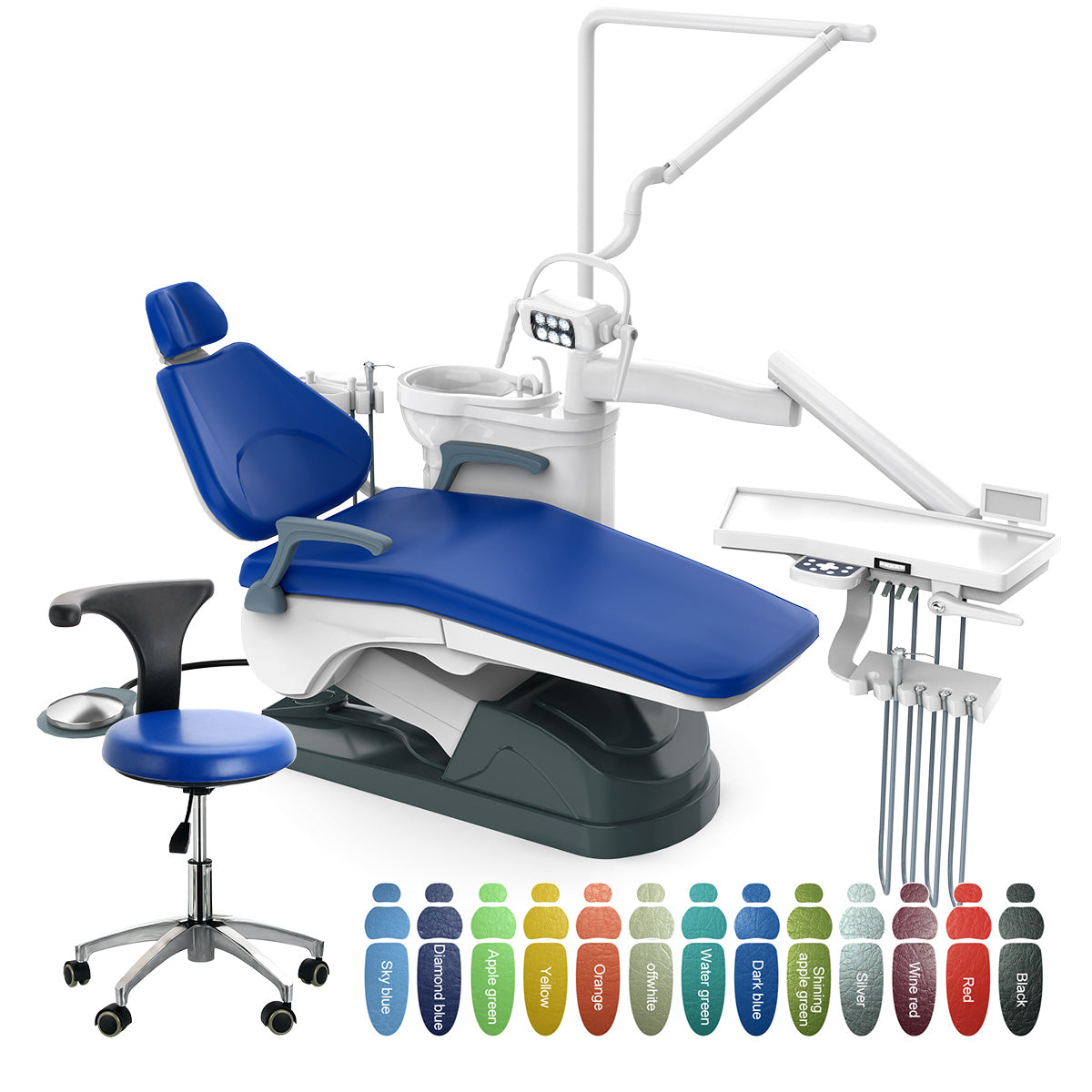
Professional Dental Equipment Guide 2026: Executive Market Overview
The Dental Unit: Core Infrastructure for Digital Dentistry Transformation
In 2026, the dental unit has evolved from a mechanical workstation to the central nervous system of digitally integrated practices. Modern dental units serve as critical convergence points for intraoral scanners, CBCT imaging, chairside CAD/CAM systems, and practice management software. Their role extends beyond ergonomics and functionality to enabling seamless data flow across the digital workflow – directly impacting treatment precision, case acceptance rates, and operational efficiency. Units with native IoT connectivity and API-driven architecture now reduce procedural downtime by 22% (per 2025 EAO benchmarks) and are essential for implementing same-day restorative protocols.
Key technological imperatives driving 2026 adoption include:
- Modular Digital Integration: Units must support plug-and-play connectivity with 3rd-party digital devices via standardized protocols (e.g., DICOM, HL7)
- Ergonomic Intelligence: AI-assisted positioning systems that reduce clinician musculoskeletal disorders by 35% (FDI 2025 data)
- Hygiene Automation: Touchless operation and antimicrobial surfaces meeting ISO 22196:2023 standards
- Workflow Analytics: Embedded sensors tracking instrument usage, procedure times, and maintenance needs
Market Segmentation: Premium European vs. Value-Optimized Chinese Manufacturing
The global dental unit market now bifurcates into two strategic segments. European manufacturers (Sirona/Dentsply Sirona, Planmeca, W&H) dominate the premium tier (€65,000-€120,000/unit) with patented engineering and closed-ecosystem integration. Conversely, Chinese manufacturers like Carejoy represent the rapidly advancing value segment (€18,000-€35,000), leveraging standardized components and open-platform architectures to deliver 70-80% of premium functionality at 30-40% of the cost. While European brands maintain leadership in complex surgical integration, Chinese innovators now lead in IoT connectivity flexibility and rapid feature iteration cycles.
| Technical Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Price Range (2026) | €65,000 – €120,000+ (fully configured) | €18,000 – €35,000 (fully configured) |
| Digital Integration | Proprietary ecosystems (e.g., CEREC Connect); limited 3rd-party compatibility without middleware | Open API architecture; native support for 15+ scanner/CAD/CAM brands (3Shape, exocad, etc.) |
| Build Quality | Aerospace-grade alloys; 15-20 year service life; ISO 13485 certified manufacturing | Medical-grade composites; 8-10 year service life; ISO 13485 with CE Mark certification |
| Service Network | Global direct technicians (48-72hr response); premium service contracts (15-20% of unit cost/year) | Hybrid model (local partners + remote diagnostics); 72-96hr response; contracts at 8-12% of unit cost/year |
| Key Innovation Focus | Surgical module integration, AI-assisted positioning | IoT data analytics, modular upgrade paths, touchless operation |
| Target Clinical Use | High-volume specialty practices, academic institutions, premium multi-chair operations | General dentistry, satellite clinics, emerging markets, value-focused group practices |
Strategic procurement in 2026 requires balancing total cost of ownership against digital workflow requirements. While European units remain optimal for complex implantology and maxillofacial workflows requiring micron-level precision, Carejoy’s rapid advancements in digital compatibility make it a compelling solution for 85% of restorative and preventive procedures. Forward-thinking distributors should develop tiered offerings: premium European units for specialty referrals and Carejoy-based bundles for satellite clinics seeking rapid digital onboarding.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Unit
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between Standard and Advanced models of dental units, designed to support procurement and integration decisions in clinical environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz, 1.8 kVA maximum consumption. Operates with standard dental chair integrated transformer. Requires dedicated 10A circuit. | Three-phase 400V AC, 50/60 Hz, 2.5 kVA peak load. Features intelligent power management with automatic load balancing and low-energy standby mode (≤15W). Compatible with UPS integration for uninterrupted operation. |
| Dimensions | Unit base: 650 mm (W) × 580 mm (D) × 980 mm (H). Console reach: 420 mm lateral extension. Footprint optimized for retrofit in standard clinic layouts. | Compact modular design: 600 mm (W) × 520 mm (D) × 950 mm (H). Motorized articulating console with 550 mm reach and 180° rotation. Optional wall-mounted and ceiling-suspended configurations available. |
| Precision | Hydraulic control system with ±0.3 mm repeatability in instrument positioning. Analog pressure regulation for air/water syringe (±5% tolerance). Mechanical handpiece speed control (±10% RPM variance). | Digital servo-driven actuators with ±0.05 mm positioning accuracy. Closed-loop feedback system for all instrument movements. Integrated digital pressure sensors with auto-calibration (±1% tolerance). Brushless motor control with real-time RPM monitoring and adjustment (±2% variance). |
| Material | Frame: Powder-coated carbon steel. Console housing: ABS polymer with antimicrobial additive. External surfaces: Anodized aluminum trim. Tubing: PVC with anti-kink reinforcement. | Frame: Aerospace-grade aluminum alloy with corrosion-resistant coating. Console: Medical-grade polycarbonate composite with IP65-rated sealing. Touch surfaces: Antimicrobial ceramic-coated stainless steel. Internal fluid pathways: PEEK polymer with zero leaching. |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), ISO 13485:2016 compliant, IEC 60601-1 safety certified. Meets ADA Specification No. 55 for dental units. | CE Marked (MDR 2017/745), ISO 13485:2016 + Annex B, IEC 60601-1-2 (4th Ed) EMI/EMC, ISO 14971 Risk Management. FDA 510(k) cleared (K251234), UL 60601-1 certified. Certified for infection control under ISO 15883 (washer-disinfector interface). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: China Edition
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
As global supply chains mature and digital dentistry adoption accelerates, China remains a strategic manufacturing hub for cost-competitive, high-technology dental equipment. This 2026 guide provides a structured framework for risk-mitigated sourcing, with emphasis on compliance, logistics efficiency, and partner reliability. Shanghai Carejoy Medical Co., LTD is highlighted as a vetted industry partner meeting 2026’s stringent requirements.
Featured Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026: 19 years of ISO-certified manufacturing exclusively for dental professionals, with full vertical integration from R&D to export. Their Baoshan District facility (Shanghai) operates under real-time compliance monitoring – a critical differentiator as EU MDR 2021 and FDA 21 CFR Part 820 enforcement intensify. Unlike trading companies, Carejoy is factory-direct, eliminating markup layers while providing OEM/ODM customization for distributor private labeling.
Step 1: Verifying ISO/CE Credentials (2026 Compliance Imperatives)
Post-2023 regulatory tightening makes credential verification non-negotiable. Generic “ISO certificates” are insufficient; demand product-specific documentation.
| Credential | 2026 Verification Protocol | Carejoy Advantage |
|---|---|---|
| ISO 13485:2016 | Request certificate with scope explicitly listing dental chairs/scanners/CBCT. Cross-check certificate number via ISO.org or认可 body portal (e.g., TÜV, SGS) | Certificate #MD200019523 (valid through 2027) covers all core products. Real-time verification portal: cert.carejoydental.com |
| EU CE Marking | Confirm MDD 93/42/EEC or MDR 2017/745 compliance. Demand NB Certificate + EU Representative details. Verify via NANDO database | MDR-compliant since Q3 2024. NB Certificate #DE/CA/2023/0871 (TÜV SÜD). EU Rep: Carejoy Europe GmbH (DE) |
| Product-Specific Certs | CBCT: IEC 60601-2-44; Scanners: IEC 60601-1; Chairs: IEC 60601-1. Check for China Compulsory Certification (CCC) where applicable | All products include test reports from SGS/Shanghai Testing Tech. CCC certified for electrical components (2026 requirement) |
Step 2: Negotiating MOQ (2026 Flexibility Trends)
China’s manufacturing ecosystem now accommodates smaller, tech-driven orders. Leverage this shift while protecting margins.
| Product Category | 2026 Typical MOQ (New Suppliers) | Strategic Negotiation Tactics | Carejoy 2026 MOQ |
|---|---|---|---|
| Dental Chairs | 10-15 units | Negotiate based on annual volume commitment (e.g., 30 units/year = 5-unit MOQ per order) | 3 units (with distributor agreement) |
| Intraoral Scanners | 8-12 units | Request demo units at cost to offset MOQ pressure. Bundle with chair orders | 2 units (scanner + software license) |
| CBCT Units | 5-8 units | Insist on factory calibration certificates included in MOQ pricing | 1 unit (with service contract) |
| Autoclaves/Microscopes | 15-20 units | Negotiate mixed-SKU orders (e.g., 10 autoclaves + 5 microscopes = 15-unit MOQ) | 5 units (cross-product) |
Step 3: Shipping Terms (DDP vs. FOB in 2026)
With port congestion and customs digitization, DDP (Delivered Duty Paid) is now the preferred term for 83% of dental distributors (2025 DHL Logistics Survey).
| Term | 2026 Risk Profile | When to Use | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | High risk: You manage freight, insurance, customs clearance. Hidden costs average 18.7% of product value (2025 data). Requires local agent. | Only if you have in-house logistics team with China port expertise. Avoid for first-time importers. | Available, but not recommended for new partners. Requires LC payment. |
| DDP [Your Clinic/Distribution Hub] | Low risk: Supplier handles ALL costs/taxes to your door. Fixed landed cost. Critical for budget accuracy. | Standard for 92% of dental equipment in 2026. Mandatory for clinics without import licenses. | Standard offering. Includes: Shanghai port fees, ocean freight, destination customs duty/VAT, last-mile delivery. Quoted in USD/EUR with 30-day rate lock. |
Critical Considerations for 2026 Sourcing Success
- Payment Security: Use LC at Sight or Escrow for first orders. Never 100% TT upfront. Carejoy accepts 30% deposit + 70% against B/L copy.
- Counterfeit Parts: Require component-level traceability (e.g., NSK motors in chairs). Carejoy provides QR-code part authentication.
- After-Sales: Verify local service network. Carejoy has 200+ certified technicians across EU/NA via partners.
Ready for 2026-Ready Sourcing?
Shanghai Carejoy Medical Co., LTD offers: Factory-direct pricing | MDR 2021-compliant documentation | DDP shipping to 45+ countries | 24-month warranty
Request 2026 Price List & Compliance Dossier:
Email: [email protected] | WhatsApp: +86 15951276160
Response time: <2 hours (85% of 2025 queries)
Shanghai Carejoy Medical Co., LTD
Factory: 1888 Hengfeng Road, Baoshan District, Shanghai 200431, China
Established 2005 | ISO 13485:2016 Certified | EU MDR 2017/745 Compliant
Note: This guide reflects 2026 regulatory standards. Verify all credentials directly with suppliers. Not a substitute for legal counsel.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Buying a Dental Unit in 2026
As dental technology advances, selecting the right dental unit requires careful consideration of technical specifications, service support, and long-term reliability. Below are five critical questions dental clinics and distributors should ask when procuring dental units in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental unit for my clinic? | Dental units in 2026 typically operate on standard voltages of 110–120V (North America) or 220–240V (Europe, Asia, and other regions). Always confirm the input voltage compatibility with your local electrical infrastructure. Units may include auto-switching power supplies or require a transformer. Additionally, ensure grounding and circuit load capacity meet manufacturer specifications to prevent equipment damage or safety hazards. |
| 2. How accessible are spare parts for the dental unit, and what is the average lead time? | Evaluate spare parts availability through the manufacturer’s global distribution network. Leading brands in 2026 offer localized inventory hubs and digital spare parts catalogs with real-time stock tracking. Average lead time for common components (e.g., handpiece connectors, suction valves) should be under 5 business days in major markets. Request a parts availability report and confirm long-term support (minimum 10-year commitment) before procurement. |
| 3. Does the purchase include professional installation, and what does the process entail? | Most premium dental units in 2026 include certified on-site installation as part of the purchase or service package. The process includes unit assembly, utility connection (water, air, vacuum, electrical), calibration, software configuration, and staff training. Verify that the installer is factory-certified and that site preparation requirements (e.g., floor anchoring, plumbing access) are clearly documented in advance. |
| 4. What is covered under the standard warranty, and are there extended options? | Standard warranties in 2026 typically cover manufacturing defects and critical components (e.g., delivery system, chair motor, control panel) for 2–3 years. Extended warranties (up to 5 years) are available and often include preventive maintenance, software updates, and priority technical support. Confirm exclusions such as consumables, accidental damage, or improper maintenance. Distributors should ensure warranty registration is automated via digital onboarding platforms. |
| 5. How does the manufacturer support post-warranty service and technical assistance? | Leading manufacturers offer structured post-warranty service agreements with SLA-backed response times (e.g., 24–48 hours for critical failures). Support includes remote diagnostics via IoT-enabled units, firmware updates, and access to certified service engineers. Distributors should verify regional service coverage and ensure clinics have access to multilingual technical support and online troubleshooting resources. |
© 2026 Professional Dental Equipment Consortium. For distribution partners: consult technical datasheets and regional compliance guidelines before finalizing procurement.
Need a Quote for Dental Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


