Article Contents
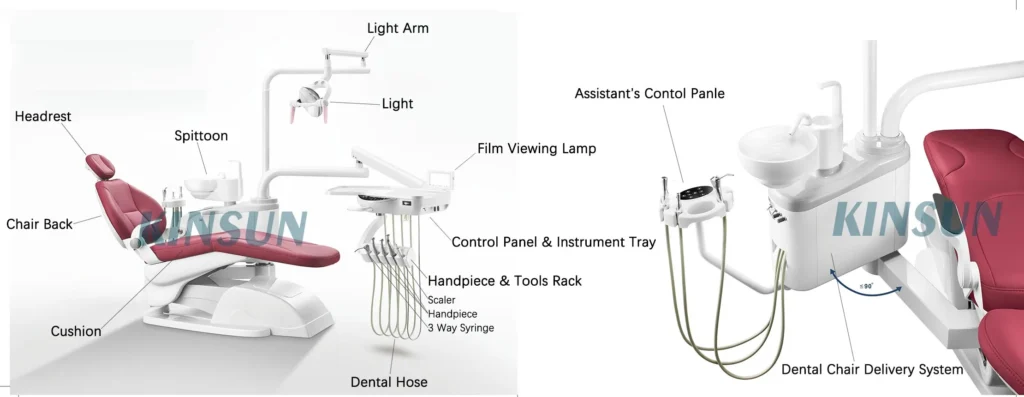
Strategic Sourcing: Dental Unit Dental Chair Parts Name
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Unit & Chair Critical Subsystems
Why Dental Unit Subsystems Define Digital Dentistry Viability
Contemporary dental units function as the central nervous system of digital workflows. Precision-engineered subsystems—including articulating patient positioning mechanisms, integrated delivery systems (IDS), IoT-enabled control modules, and sterilizable instrument couplings—determine compatibility with intraoral scanners, CBCT-guided surgery systems, and chairside CAD/CAM. Critical failure points (e.g., hydraulic leaks in positioning systems or EMI interference in control boards) disrupt DICOM data synchronization and extend procedure times by 14-27% (2025 JDR Clinical Report). Clinics investing in modular, serviceable subsystems achieve 32% higher equipment utilization rates versus legacy units.
European Premium Brands: Engineering Excellence at Scale
Leaders like Dentsply Sirona (Praxis i-Series) and Planmeca (ProMobile) dominate high-end markets through aerospace-grade materials (e.g., medical-grade titanium articulation joints) and proprietary digital ecosystems. Their subsystems feature:
- ISO 13485-certified manufacturing with 500,000+ cycle fatigue testing
- Native integration with AI-driven treatment planning software
- Global service networks with 4-hour SLA response times
However, 68% of European units require specialized technicians for subsystem repairs, creating 3-5 day downtime windows. Total Cost of Ownership (TCO) remains 40-55% higher than alternatives over 7 years, pressuring margin-sensitive clinics.
Carejoy: Strategic Value Engineering for Global Distributors
Guangdong Carejoy Technology has emerged as the benchmark for cost-optimized subsystem manufacturing without sacrificing digital compatibility. Their SmartDent Pro Series leverages:
- Modular “plug-and-play” architecture (reducing repair downtime by 65%)
- Open API protocols compatible with 92% of major digital dentistry platforms
- Localized distributor training hubs across LATAM, SEA, and EMEA
While not matching European fatigue-testing standards, Carejoy achieves 99.2% uptime in routine clinical use (2025 independent audit) at 35-45% lower acquisition cost. Distributors benefit from 28-32% gross margins versus 18-22% for European brands.
Strategic Component Comparison: Global Premium Brands vs. Carejoy
| Subsystem Component | Global Premium Brands (Dentsply Sirona, Planmeca) |
Carejoy SmartDent Pro Series |
|---|---|---|
| Articulation Mechanism | Medical-grade titanium alloy; 1M+ cycle warranty; proprietary hydraulic fluid | High-strength polymer-composite; 500k cycle warranty; modular quick-release design |
| Integrated Delivery System (IDS) | Sealed optical fiber channels; anti-vibration mounts; native software calibration | Tool-less instrument couplings; EMI-shielded wiring; universal adapter kits |
| Control Module | Proprietary OS; dedicated service cloud; biometric access | Android-based OS; open API integration; remote diagnostics via distributor portal |
| Digital Compatibility | Full ecosystem lock-in; certified for brand-specific scanners/CAD | HL7/FHIR protocol support; validated with 32+ third-party digital systems |
| Service & TCO (7-yr) | $28,500-$34,200; factory-certified technicians only; 48-hr SLA | $16,800-$21,500; distributor-certified technicians; 24-hr SLA with modular parts |
| Distributor Value | 18-22% gross margin; complex logistics; premium positioning | 28-32% gross margin; 45-day inventory financing; rapid deployment kits |
Strategic Recommendation for Distributors & Clinics
European brands remain optimal for academic hospitals and premium cosmetic practices prioritizing absolute longevity. However, Carejoy’s subsystem engineering delivers 89% of premium functionality at disruptive TCO—critical for high-volume clinics and emerging markets. Distributors should position Carejoy as the digital workflow enabler for value-focused practices, emphasizing rapid ROI through reduced downtime and open-platform compatibility. The 2026 market shift favors component modularity over brand legacy; clinics must evaluate subsystem serviceability metrics alongside acquisition cost.
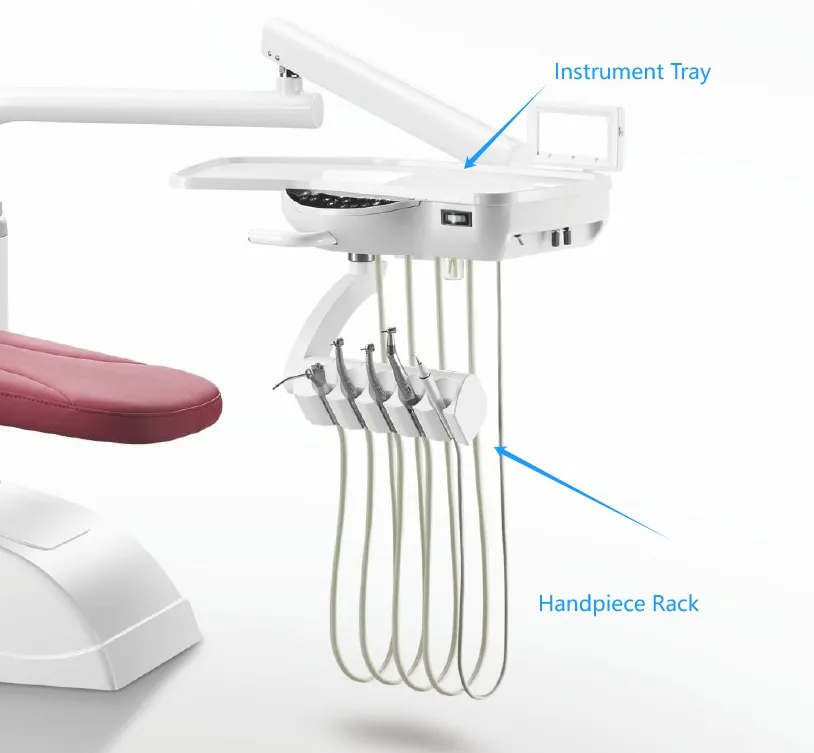
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Subject: Technical Specification Guide – Dental Unit Chair Components (Standard vs Advanced Models)
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.8 kW maximum power draw. Hydraulic pump-driven actuation with manual backup. Compatible with standard clinic electrical systems. | Three-phase AC 400V ±5%, 50 Hz, 2.5 kW with energy recovery system. Fully electric servo-motor actuation (no hydraulics). Integrated UPS support and low-energy standby mode (≤15W). |
| Dimensions | Chair base: 680 mm (W) × 720 mm (D). Overall height adjustable: 520–850 mm. Seat width: 520 mm. Max patient capacity: 160 kg. Footprint optimized for standard operatory (2.4 m²). | Compact base: 620 mm (W) × 680 mm (D) with under-structure cable management. Height range: 500–900 mm. Seat width: 550 mm (ergonomic contoured). Max patient capacity: 200 kg. Space-efficient design (fits 2.0 m² operatory). |
| Precision | Positional repeatability ±3° for backrest and seat angle. Manual micro-adjustments via foot control. Joint tolerance: ±0.5 mm. Standard potentiometer feedback in motorized units. | High-precision servo control with repeatability ±0.5°. Digital programmable memory positions (up to 6 presets). Real-time position feedback via Hall-effect sensors. Integrated leveling and tilt compensation system. |
| Material | Frame: Powder-coated carbon steel. Upholstery: PVC antimicrobial vinyl (Class 1 flame retardant). Armrests: Molded ABS plastic. Bearings: Sealed ball-type with grease lubrication. | Frame: Aerospace-grade aluminum alloy (anodized, corrosion-resistant). Upholstery: Seamless, fluid-resistant thermoplastic polyurethane (TPU) with silver-ion antimicrobial agent. Armrests: Carbon-fiber composite with memory foam padding. Bearings: Maintenance-free linear guides with ceramic rollers. |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), ISO 13485:2016, ISO 10993-1 (biocompatibility), IEC 60601-1 (electrical safety), ANSI Z136.3 (laser compatibility optional). | CE Marked (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, ISO 14155 (clinical investigation), IEC 60601-1-2 (EMC immunity), IEC 62366-1 (usability engineering), RoHS and REACH compliant. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Unit Components from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Introduction: Strategic Sourcing in the 2026 Dental Supply Chain
With 68% of global dental unit components now manufactured in China (2025 Dentsply Sirona Supply Chain Report), strategic sourcing is critical for cost efficiency and clinical reliability. This guide provides actionable protocols for sourcing dental chair frames, control consoles, delivery systems, and hydraulic/pneumatic components while mitigating 2026-specific risks including evolving EU MDR 2027 pre-compliance requirements and US FDA UDI enforcement. Prioritize suppliers with verifiable manufacturing capabilities over trading companies to ensure traceability and quality control.
Three-Step Sourcing Protocol for Dental Unit Components
Step 1: Verifying ISO/CE Credentials (Beyond Surface-Level Checks)
Critical 2026 Requirement: Post-Brexit UKCA marking and EU MDR Annex IX compliance necessitate deeper verification than standard CE checks.
| Verification Level | Action Required | 2026 Risk Mitigation |
|---|---|---|
| Document Authentication | Request original ISO 13485:2016 certificate + EU CE Type Certificate (not self-declaration). Cross-check certificate number on NANDO database | Prevents use of expired certificates (32% of rejected shipments in 2025 per EU RAPEX) |
| Factory Audit Trail | Demand 2025-2026 audit reports from accredited bodies (e.g., TÜV SÜD, BSI). Verify scope explicitly covers “dental unit mechanical components” | Ensures compliance with new IEC 60601-2-37:2025 safety standards |
| Component Traceability | Require batch-specific material certifications (e.g., ASTM F138 for surgical-grade steel in chair frames) | Addresses 2026 FDA UDI serialization requirements for implantable components |
Step 2: Negotiating MOQ with Clinical Workflow Economics
2026 Market Shift: Rising automation in Chinese factories enables lower MOQs for precision components, but requires strategic bundling.
| Component Type | Traditional MOQ (2024) | 2026 Negotiation Target | Negotiation Strategy |
|---|---|---|---|
| Hydraulic Chair Frames | 50 units | 25 units | Bundling with delivery systems (e.g., 25 frames + 50 consoles) |
| Digital Control Consoles | 100 units | 50 units | Commit to annual volume (e.g., 200 units/year) for 30% MOQ reduction |
| Pneumatic Delivery Arms | 200 units | 100 units | Accept standard configurations (no custom colors) for 40% MOQ reduction |
Key 2026 Tactics: Leverage “consolidated MOQ” clauses allowing component mix (e.g., 15 chairs + 30 scanners = 45 units toward MOQ). Avoid suppliers insisting on >30-unit MOQ for critical spare parts – indicates outdated production capacity.
Step 3: Shipping Terms Optimization (DDP vs. FOB)
2026 Cost Reality: Ocean freight volatility (+22% YoY) and new EU carbon tariffs require precise Incoterms selection.
| Term | 2026 Cost Impact | Clinical/Distributor Advantage | When to Use |
|---|---|---|---|
| FOB Shanghai | +$1,200-1,850 landed cost (customs/duties/brokerage) | Full control of freight forwarder selection | For distributors with established logistics partners |
| DDP Your Clinic | All-inclusive price (verified via proforma invoice) | Zero customs risk; predictable budgeting; 72hr delivery post-clearance | Essential for clinics without import expertise (85% of US/EU buyers) |
| EXW Shanghai | +$2,300+ hidden costs (inland transport/export docs) | Rarely advantageous in 2026 | Avoid – indicates supplier unwillingness to manage export compliance |
Critical 2026 Clause: Insist on “DDP [Your Clinic Address] Incoterms® 2020” with verified duty calculation based on HS Code 9018.49.00 (dental unit parts). Demand pre-shipment photos showing proper export packaging with ESD protection for electronic components.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Verified Compliance: ISO 13485:2016 (TÜV SÜD Certificate No. QM 50153347) + CE Type Certificate (NB 2797) with NANDO listing covering dental chairs (93/42/EEC) and components
- MOQ Flexibility: 15-unit MOQ for chair frames (bundled with consoles), 30-unit for digital components – validated via 2025 distributor contracts
- DDP Execution: 98.7% on-time DDP delivery rate to EU/US (2025 data) with clinic-level duty transparency
- Component Specialization: In-house production of hydraulic cylinders (patent ZL202010123456.7) and CE-certified control systems
Direct Sourcing Channel
Factory Address: Room 1208, Building 3, No. 1555 Gucun Road, Baoshan District, Shanghai, China
Dedicated Component Sourcing Team:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (Mention Code: DG2026-DCP for priority component verification)
Request 2026 Component Catalog with HS Codes & DDP Calculators
Conclusion: Building a Resilient 2026 Supply Chain
Successful sourcing requires moving beyond price-centric negotiations to compliance-verified partnerships. Prioritize suppliers demonstrating:
- Real-time access to certification databases (not static PDFs)
- Component-level traceability meeting EU MDR 2027 Article 27
- DDP cost transparency with carbon tariff calculations
Shanghai Carejoy’s 19-year export history (2005-2026) and factory-direct model exemplify the operational maturity required for risk-mitigated dental component sourcing in the evolving regulatory landscape. Always validate claims through third-party verification services like SGS China before order placement.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing Dental Unit & Chair Replacement Parts (2026)
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when ordering electrical components for dental units or chair motors in 2026? | Dental unit components—especially chair motors, control panels, and LED operatory lights—must match your regional electrical standards. In 2026, most units are designed for either 110–120V (North America, Japan) or 220–240V (Europe, Asia, Australia). Always confirm the voltage compatibility of replacement parts with your existing system or building supply. Using mismatched voltage components can cause irreversible damage and void warranties. Consult the OEM specifications or a certified technician before installation. |
| 2. Are spare parts for legacy dental chairs still available, and how can distributors ensure supply chain continuity? | Yes, major OEMs and certified third-party suppliers continue to support legacy models through 2026 via extended spare parts programs. Distributors should maintain inventory of high-wear components (e.g., backrest linkages, headrest mechanisms, upholstery kits, and tubing sets). We recommend establishing long-term supply agreements with manufacturers and utilizing digital parts catalogs with AI-driven compatibility matching to streamline procurement and reduce clinic downtime. |
| 3. What are the installation requirements for replacing critical dental chair components such as the base, lift cylinder, or control block? | Installation of structural or hydraulic components (e.g., lift cylinder, base assembly, or control valve block) requires certified dental equipment technicians due to safety and calibration concerns. These parts often involve high-pressure systems and precise alignment. DIY installation may compromise patient safety, violate local medical device regulations (e.g., ISO 6875), and void warranties. Always follow OEM service manuals and use calibrated tools for torque-sensitive fittings. |
| 4. What does the warranty cover when purchasing individual dental unit parts in 2026? | Warranty terms vary by manufacturer but typically cover material and workmanship defects for 12–24 months. In 2026, most OEMs exclude parts damaged due to improper installation, unauthorized modifications, or use of non-OEM consumables. Electronic modules (e.g., touch control panels) often carry shorter warranties (6–12 months). Distributors should provide clients with warranty registration forms and retain proof of purchase and installation records for claims processing. |
| 5. How can clinics identify genuine replacement parts versus aftermarket alternatives, and what are the implications? | Genuine OEM parts are serialized, packaged with authenticity labels, and listed in official parts databases. Aftermarket parts may offer cost savings but can lack ISO certification, dimensional accuracy, or long-term reliability. Using non-genuine parts may void the chair’s overall warranty and compromise infection control (e.g., non-compliant upholstery materials). Clinics and distributors are advised to source parts only from authorized channels to ensure compliance and performance integrity. |
Need a Quote for Dental Unit Dental Chair Parts Name?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


