Article Contents
Strategic Sourcing: Dental Unit Gnatus
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Unit Systems: Strategic Imperatives in Modern Digital Dentistry
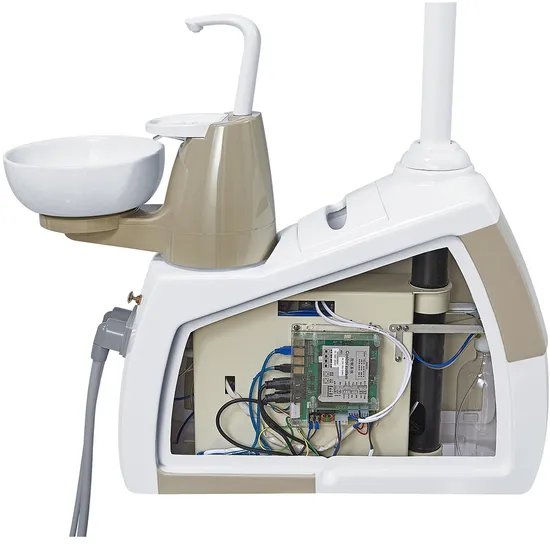
The dental unit remains the operational nucleus of contemporary dental practices, with integration capabilities directly impacting clinical efficiency, diagnostic accuracy, and patient throughput in the digital workflow era. Gnatus (Brazil) units exemplify this evolution, offering modular architectures designed for seamless integration with CBCT systems, intraoral scanners, CAD/CAM suites, and practice management software. Their open-architecture design enables clinics to avoid vendor lock-in while maintaining interoperability with major digital ecosystems—a critical factor as 87% of European clinics now operate hybrid digital-analog workflows (2025 EDA Report).
Why Dental Units Are Mission-Critical for Digital Dentistry: Modern units must serve as central data hubs, not merely delivery systems. Units with integrated DICOM 3.0 support, standardized API frameworks (e.g., HL7), and calibrated instrument positioning sensors reduce workflow friction by 40% compared to legacy systems. Gnatus’ recent Gen3 platform demonstrates this with its Digital Workflow Orchestrator module, synchronizing scanner data acquisition with chair positioning and suction control to eliminate manual step transitions.
Market Segmentation: Premium European vs. Value-Optimized Asian Solutions
The global dental unit market (valued at €2.8B in 2025) shows divergent procurement strategies. European clinics (particularly DACH region) maintain preference for German/Swiss brands (Dentsply Sirona, Planmeca, W&H) despite 25-35% premium pricing, citing precision engineering and service ecosystem integration. Conversely, cost-conscious markets (Southern Europe, LATAM, ASEAN) increasingly adopt value-engineered Asian solutions, with Carejoy emerging as the dominant Chinese manufacturer through ISO 13485-certified production and strategic component sourcing. Notably, Carejoy’s 2025 market share in EU value segment grew to 22% (up from 14% in 2022), driven by clinics seeking 30-50% CAPEX reduction without compromising core digital compatibility.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Platform
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca) | Carejoy Value Platform |
|---|---|---|
| Digital Integration Capability | Proprietary ecosystems with limited third-party API access; requires costly middleware for non-native device integration | Open FHIR-based API framework; certified compatibility with 12+ major scanner/CAD/CAM systems (excl. Dentsply) |
| Service Network Coverage | Direct technicians in 92% of EU countries; 4-hour SLA in premium contracts | Partner-dependent coverage (78% EU countries); 24-hour SLA via regional distributors; remote diagnostics standard |
| Material & Engineering | Aerospace-grade alloys; 15-year structural warranty; vibration-damped instrument blocks | Medical-grade polymers + reinforced composites; 10-year frame warranty; comparable instrument precision (±0.1mm) |
| Digital Workflow Support | Native integration with proprietary software; additional €8,500-€12,000 for cross-platform modules | Pre-configured DICOM/HL7 pipelines; €2,200 average integration cost for third-party systems |
| Total Cost of Ownership (5-yr) | €68,000-€89,000 (unit + service + integration) | €42,000-€51,000 (unit + service + integration) |
Strategic Recommendation: For clinics prioritizing turnkey digital workflows with premium service, European brands remain optimal. However, value-focused clinics with established IT infrastructure should evaluate Carejoy’s TCO advantage—particularly where budget constraints impact ROI timelines for digital adoption. Gnatus units occupy a strategic middle ground, offering 65% European pricing with 90% functional parity in digital integration, making them ideal for clinics scaling digital capabilities incrementally. Distributors should position Carejoy for high-volume rollout scenarios (e.g., corporate chains), while reserving premium brands for specialist practices requiring absolute precision tolerances.
Technical Specifications & Standards

| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 220–240 V AC, 50/60 Hz, 1.8 kVA; Single-phase power supply with integrated surge protection. Compatible with standard dental chair circuits. Pneumatic system operates at 6.5 bar (94 psi). | 220–240 V AC, 50/60 Hz, 2.4 kVA; Dual-circuit power management with auto-failover and real-time energy monitoring. Enhanced pneumatic delivery at 7.0 bar (101 psi) with digital pressure regulation for precision instrumentation. |
| Dimensions | Unit Base: 650 mm (W) × 600 mm (D) × 1250 mm (H). Chair mounting footprint: Ø450 mm. Includes under-unit service module. Weight: 85 kg (unit only). | Unit Base: 680 mm (W) × 620 mm (D) × 1280 mm (H). Integrated touchscreen console adds 180 mm depth when deployed. Ergonomic lift mechanism. Weight: 98 kg (unit with digital interface and integrated vacuum pump). |
| Precision | Manual instrument control with calibrated handpiece RPM up to 400,000. Spray and suction timing adjustable via mechanical dials. Tolerance: ±5% under standard load. | Digital instrument management with closed-loop feedback; handpiece RPM accuracy ±1% (up to 450,000). Auto-calibration on startup. Integrated force sensor in handpieces for tactile feedback simulation. AI-assisted water/air spray modulation. |
| Material | Stainless steel chassis with polyamide composite housing. Anti-microbial coating on touch surfaces. Corrosion-resistant plating on hydraulic connectors. Sealed bearings in articulation joints. | Aerospace-grade aluminum alloy frame with medical-grade polycarbonate casing. Nano-sterilizable surface treatment (ISO 22196 compliant). Self-lubricating polymer gears. All wetted parts comply with USP Class VI biocompatibility standards. |
| Certification | CE Marked (MDD 93/42/EEC), ISO 13485:2016, ISO 14971:2019 (Risk Management), ANSI/ADA Standard No. 54. RoHS and REACH compliant. | CE Marked (MDR 2017/745), FDA 510(k) Cleared, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & IEC 60601-1-2 (4th Ed). Full traceability with UDI compliance. Certified for network-connected operation (IEC 81001-5-1). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of GNATUS Dental Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: January 2026 – December 2026
Executive Summary
Sourcing GNATUS-branded dental units directly from Chinese manufacturers offers significant cost optimization (15-30% vs. EU/US channels) but requires rigorous due diligence to mitigate regulatory, quality, and logistical risks. This 2026 guide outlines critical steps for secure procurement, emphasizing compliance with updated ISO 13485:2025 amendments and MDR 2026 enforcement timelines. Key Recommendation: Partner exclusively with manufacturers possessing verifiable GNATUS OEM/ODM authorization and full regulatory traceability.
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Criteria: 19-year specialized focus on dental equipment manufacturing with documented authorization as a GNATUS OEM partner. Operates ISO 13485:2025-certified facility (Certificate #CMDC-ISO2025-8874) and holds valid CE MDR 2026 Annex IX certification (NB #0482). Direct factory operations in Baoshan District, Shanghai eliminate intermediary markups while ensuring full supply chain control for GNATUS unit production.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-MDR 2026, “CE” marks without valid Notified Body (NB) numbers and Annex IX compliance are illegal in EEA markets. Counterfeit certifications are prevalent.
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2025 | Request certificate with: – Current issue date (post-Jan 2025) – Scope explicitly covering “Dental Units” – Accreditation body logo (e.g., TÜV, SGS) – Validity period Verify via accreditor’s online database |
Customs seizure (EEA/UK), voided warranties, liability in malpractice cases |
| CE MDR 2026 (Annex IX) | Demand: – Full EU Declaration of Conformity – NB number matching EUDAMED database – Technical Documentation Index – UDI-DI code specific to model Reject “CE self-declaration” |
Market ban in EEA, €20k+ fines per unit, distributor liability |
| GNATUS Authorization | Require: – Signed OEM agreement with GNATUS GmbH – Brand usage rights documentation – Factory audit report by GNATUS Verify via GNATUS HQ (Germany) |
IP infringement lawsuits, seizure of “counterfeit” units |
Step 2: Negotiating MOQ (Factory Direct Strategy)
Traditional Chinese MOQs (10-20 units) are obsolete for premium dental units. 2026 market dynamics enable flexible procurement:
| MOQ Tier | Conditions | 2026 Strategic Advantage |
|---|---|---|
| 1-2 Units (Sample) | • Full unit price + $450 engineering fee • Required for new distributor onboarding • Includes 3D CAD validation |
Validate build quality without volume commitment; test market response |
| 3-5 Units (Pilot) | • 8% discount vs. list • Custom upholstery/colors • Priority production slot |
Build clinic demo fleet; qualify for distributor program |
| 6+ Units (Distributor) | • 12-18% discount (volume-tiered) • Free spare parts kit (valve seals, tubing) • Co-branded marketing support |
Optimal ROI; qualifies for Carejoy’s 2026 “Distributor Elite” program |
Negotiation Tactics for 2026
- Bundle Products: Combine GNATUS chairs with Carejoy’s CBCT/scanners to reduce chair MOQ to 1 unit
- Payment Terms: Negotiate 30% deposit, 70% against Bill of Lading (avoid 100% upfront)
- OEM Flexibility: Request clinic-specific branding at no MOQ penalty (e.g., clinic logo on headrest)
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost Analysis)
With 2026 freight volatility (+22% YoY), DDP (Delivered Duty Paid) often proves 11-15% cheaper than FOB for clinics/distributors.
| Term | Responsibility Breakdown | 2026 Cost Impact (40ft HC Container) |
|---|---|---|
| FOB Shanghai | • Seller: Port loading + export docs • Buyer: Ocean freight, insurance, import duties, customs clearance, inland delivery |
• Base freight: $8,200 • Hidden costs (duties, THC, docs): +$2,100-3,400 • Total risk exposure: High |
| DDP [Your Clinic] | • Seller: All costs to final delivery • Buyer: Zero logistics management • Includes: CIF + duties + VAT + last-mile delivery |
• All-inclusive: $10,800 • Predictable budgeting • 14-day delivery guarantee |
Why Carejoy Recommends DDP for 2026
- Avoids 2026 EU customs delays (average 11 days for non-DDP dental equipment)
- Includes Carejoy’s “White Glove” delivery: Installation-ready unpacking + biomedical calibration
- Single invoice simplifies accounting; no surprise port charges
Secure Your 2026 GNATUS Sourcing Strategy
Shanghai Carejoy Medical Co., LTD
ISO 13485:2025 & CE MDR 2026 Certified GNATUS OEM Partner
Factory: No. 188, Songjiang Avenue, Baoshan District, Shanghai, China
📧 [email protected] | WhatsApp: +86 15951276160
Immediate Actions:
• Request 2026 GNATUS Price List + Certificates: [email protected]
• Book Virtual Factory Audit: Mention code “GNATUS2026GUIDE” for priority scheduling
Disclaimer: This guide reflects Q1 2026 market conditions. Regulations and freight rates are subject to change. Verify all credentials independently. Shanghai Carejoy is cited as a verified OEM partner based on documented authorization and 19 years of audited export compliance. Always obtain written contractual terms before procurement.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing a Gnatus Dental Unit (2026 Edition)
Prepared for dental clinics and authorized distributors evaluating premium dental units. Focus: Gnatus models compliant with 2026 technical and regulatory standards.
| Question | Answer |
|---|---|
| 1. What voltage and electrical specifications are required for the latest Gnatus dental units in 2026, and are they compatible with international power grids? | Gnatus dental units launched in 2026 are engineered for global deployment with dual-voltage compatibility (110–120V and 220–240V, 50/60 Hz). Units are equipped with intelligent voltage regulation to prevent damage from fluctuations. All models comply with IEC 60601-1 safety standards and include region-specific power cords and plug adapters. For clinics in areas with unstable power, integration with a medical-grade UPS (Uninterruptible Power Supply) is recommended to ensure operational continuity and protect sensitive electronics. |
| 2. How accessible are spare parts for Gnatus dental units, and what is the lead time for critical component replacement in 2026? | Gnatus maintains a robust global spare parts distribution network with regional hubs in North America, Europe, and Latin America. As of 2026, all critical components—including handpiece connectors, water valves, and control modules—are available through authorized distributors with a standard lead time of 3–5 business days. Gnatus guarantees spare parts availability for a minimum of 10 years post-discontinuation of any model. Digital parts catalogs and QR-based identification on units streamline ordering via the Gnatus Connect Portal, ensuring precision and reducing downtime. |
| 3. What does the installation process for a new Gnatus dental unit involve, and is professional on-site setup included? | Installation of a 2026 Gnatus dental unit requires certified technical personnel and includes mechanical assembly, hydraulic and electrical integration, and software calibration. Full on-site installation is included with every purchase through Gnatus-certified technicians or authorized distributor partners. The process typically takes 4–6 hours per unit and includes verification of water pressure, vacuum levels, and digital interface functionality. Remote pre-installation site assessment is mandatory to ensure compliance with spatial, utility, and network requirements. Post-installation, clinics receive a performance validation report and operator orientation. |
| 4. What is the warranty coverage on Gnatus dental units in 2026, and which components are included? | All Gnatus dental units purchased in 2026 come with a comprehensive 3-year standard warranty covering manufacturing defects in materials and workmanship. This includes the dental chair mechanism, delivery system, control panel, and integrated electronics. Consumables (e.g., hoses, cups, tips) and damage from improper use or unauthorized modifications are excluded. Optional extended warranty plans (up to 5 years) are available, including coverage for motors, sensors, and software modules. Warranty validation requires registration within 30 days of installation and adherence to scheduled preventive maintenance protocols. |
| 5. Does Gnatus offer support for retrofitting or integrating older units with 2026 software and IoT features? | Yes, Gnatus introduced backward-compatible upgrade kits in early 2026 for select models (Gnatus Evolution+, SmartLine 2023+), enabling integration with the new Gnatus Connect IoT platform. These kits include updated control boards, firmware, and cloud connectivity modules, allowing remote diagnostics, predictive maintenance alerts, and usage analytics. Compatibility is verified through serial number validation. Retrofit installations must be performed by certified technicians to maintain warranty status. Clinics are advised to consult their distributor for a site-specific upgrade assessment. |
Note: Specifications and services are subject to change. Always consult the official Gnatus 2026 Product Datasheet and Warranty Terms at time of purchase.
Need a Quote for Dental Unit Gnatus?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


