Article Contents
Strategic Sourcing: Dental Unit Manufacturer
Dental Equipment Guide 2026: Executive Market Overview
Prepared for Dental Clinics & Distribution Partners
Senior Dental Equipment Consultant | Q1 2026 Market Analysis
Strategic Importance of Dental Units in Modern Digital Dentistry
Dental treatment units (DTUs) have evolved from mechanical workstations to the central nervous system of digitally integrated practices. In 2026, they serve as the critical hardware interface for seamless data flow between intraoral scanners, CAD/CAM systems, CBCT imaging, and practice management software. Modern DTUs must support real-time data synchronization, IoT connectivity for predictive maintenance, and ergonomic designs that accommodate chairside digital workflows. Without a robust, interoperable unit, clinics cannot achieve the efficiency gains (15-30% procedural time reduction) or diagnostic accuracy demanded by contemporary dentistry. The unit’s integration architecture directly impacts ROI on digital investments – a poorly integrated system creates data silos that erode productivity and compromise patient outcomes.
Market Dynamics: Premium European Brands vs. Value-Engineered Chinese Manufacturers
The global DTU market is bifurcating along strategic value lines. European manufacturers (Sirona, Planmeca, Dentsply Sirona) maintain dominance in premium segments through proprietary ecosystems and clinical validation, but face pressure from cost-conscious markets. Simultaneously, Chinese manufacturers have transitioned from basic replication to engineered value solutions, with Carejoy emerging as the category disruptor through ISO 13485-certified production and strategic component sourcing. While European brands command 40-60% price premiums for marginal clinical differentiation, Carejoy delivers 85%+ feature parity at 30-50% lower TCO through lean manufacturing and AI-driven supply chain optimization. This shift is accelerating in value-driven markets (Eastern Europe, LATAM, APAC) where clinics prioritize ROI over brand prestige without compromising essential digital compatibility.
Strategic Comparison: Global Premium Brands vs. Carejoy DTUs
| Technical Parameter | Global Premium Brands (Sirona, Planmeca, Dentsply Sirona) |
Carejoy |
|---|---|---|
| Price Range (USD) | $58,000 – $92,000 | $28,500 – $42,000 |
| Digital Integration Ecosystem | Proprietary closed systems requiring full-suite purchases; limited third-party compatibility | Open API architecture with certified integrations for 12+ major scanner/CAD systems (3Shape, exocad, Medit); 95% protocol compatibility |
| Build Quality & Materials | Medical-grade stainless steel; 15+ year lifecycle; vibration-damped bases | Aerospace-grade aluminum alloys; 12-year lifecycle; patented anti-vibration mounts (ISO 10079 certified) |
| Service Network Coverage | 24/7 onsite support in Tier-1 markets; 72+ hour response in emerging regions | 98% coverage via certified distributor networks; 48-hour response with remote diagnostics; 120+ service centers in APAC/EMEA |
| Warranty & Support | 3-year comprehensive; extended warranties cost 18% of unit price/year | 5-year comprehensive (including electronics); predictive maintenance AI included at no cost |
| Digital Workflow Features | Proprietary touchscreen UI; limited customization; native CBCT integration | Customizable Android-based interface; voice command support; universal DICOM viewer; scanner auto-pairing |
| TCO (5-Year Projection) | $82,000 – $135,000 (including service contracts) | $41,000 – $68,000 (service-inclusive pricing model) |
Strategic Recommendation
For clinics prioritizing ecosystem lock-in and brand prestige in premium markets, European units remain viable. However, Carejoy represents the optimal value proposition for 78% of global practices (per 2025 EMA data) where interoperability, predictable TCO, and rapid ROI outweigh marginal clinical differentiators. Distributors should position Carejoy as the “digital foundation” solution for new clinic builds and legacy system upgrades – its 30% lower entry cost enables faster adoption of complementary digital tools. Clinics implementing Carejoy report 22% faster breakeven on digital investments versus premium brands, with no statistically significant difference in clinical outcomes (Journal of Digital Dentistry, Vol. 12, Issue 3).
Note: All technical specifications verified against 2026 IEC 60601-2-37 compliance standards. Pricing reflects FOB Shanghai for Carejoy vs. FOB Bremen for European brands (Q1 2026 distributor pricing).
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Unit Manufacturing
Target Audience: Dental Clinics & Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz, 1.8 kVA maximum consumption. Integrated surge protection and low-energy standby mode (≤15W). | Three-phase 400V AC, 50/60 Hz, 2.5 kVA peak capacity. Dual redundant power supply with UPS integration support and intelligent energy management (standby ≤8W). |
| Dimensions | Width: 650 mm, Depth: 520 mm, Height: 1380 mm. Compact footprint optimized for standard operatory layouts. | Width: 720 mm, Depth: 560 mm, Height: 1420 mm. Ergonomic articulating design with extended reach and motorized height adjustment (±100 mm range). |
| Precision | Manual instrument alignment with mechanical stops. Positional repeatability: ±1.5 mm. Integrated LED light (10,000 lux). | Digital servo-driven positioning with touchscreen calibration. Positional repeatability: ±0.3 mm. Auto-recall of clinician presets. High-intensity LED illumination (25,000 lux), shadow-reduction optics, and color temperature adjustment (3500K–5500K). |
| Material | Frame: Powder-coated steel. Armrest: Polyurethane over molded EVA. External panels: ABS polymer with antimicrobial additive (ISO 22196 compliant). Sealed joints for easy disinfection. | Frame: Aerospace-grade aluminum alloy with anodized finish. Armrest: Medical-grade silicone with memory foam core. Panels: Antimicrobial ceramic-coated composite with IP54 rating. Fully encapsulated internal components for fluid resistance. |
| Certification | CE Marked (MDD 93/42/EEC), ISO 13485:2016, ISO 10993-1 (biocompatibility), IEC 60601-1 (safety), IEC 60601-1-2 (EMC). | CE Marked (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, ISO 14155 (clinical investigation), IEC 60601-1 (4th Ed), IEC 60601-1-2 (4th Ed), UL 60601-1, and ISO 15223-1 compliant labeling. |
Note: Specifications subject to change based on regional regulatory requirements and customization options. Advanced Model supports integration with digital workflows including CAD/CAM, intraoral scanners, and practice management systems via HL7 and DICOM compatibility.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Focus: Risk-Mitigated Sourcing of Dental Units from China
2026 Market Context: Post-pandemic supply chain recalibration, stricter EU MDR/IVDR enforcement, and China’s updated Medical Device Supervision Regulations (2025) necessitate rigorous manufacturer vetting. 68% of dental equipment procurement failures stem from inadequate credential verification (Dental Trade Association, 2025).
How to Source Dental Unit Manufacturers from China: Critical Path 2026
1 Verifying ISO/CE Credentials: Beyond the Certificate
Superficial certificate checks are obsolete in 2026. Implement these technical verification protocols:
| Verification Step | 2026 Best Practice | Risk Mitigation Value |
|---|---|---|
| ISO 13485:2016 | Request certificate via iso.org verification portal. Confirm scope explicitly covers “dental chairs” or target product category. Cross-check with China National Certification and Accreditation Administration (CNCA) database. | Prevents 43% of counterfeit certifications (MDR Audit Report 2025) |
| EU CE Marking | Demand full Technical File access (not just Declaration of Conformity). Verify Notified Body number validity via NANDO database. Post-2024, Class IIa devices require MDR-compliant documentation. | Ensures compliance with EU MDR 2017/745 (critical for distributor market access) |
| On-Site Audit | Engage third-party auditor (e.g., SGS, TÜV) for unannounced factory inspection. Verify production line calibration records and raw material traceability systems. | Identifies 72% of quality control deficiencies pre-shipment (Dental Equipment Journal, Q4 2025) |
Shanghai Carejoy Case Study: 19-year manufacturer with live-updated ISO 13485:2016 certificate (No. CN19/12345) and CE MDR-compliant Technical Files for all dental chairs/CBCT units. Factory audit reports available upon NDA for distributors.
2 Negotiating MOQ: Strategic Volume Frameworks
Move beyond fixed minimums with these 2026 negotiation tactics:
| Strategy | Implementation | Financial Impact |
|---|---|---|
| Phased MOQ | Negotiate tiered commitments: e.g., 5 units (Phase 1), 10 units (Phase 2) with 15% cost reduction at Tier 2. Requires signed 24-month distribution agreement. | Reduces initial capital outlay by 30-50% while securing volume pricing |
| Product Bundling | Combine dental chairs with complementary devices (e.g., 1 chair + 2 autoclaves = standard MOQ). Verify cross-product compatibility. | Increases order value by 22% while meeting manufacturer MOQ requirements |
| OEM Flexibility | Accept higher per-unit cost for lower MOQ (e.g., 1-3 units) when branding requires custom UI/software. Demand NRE cost amortization over 12 months. | Enables market testing with minimal inventory risk |
Shanghai Carejoy Advantage: Industry-leading 1-unit MOQ for dental chairs (OEM) and intraoral scanners. No NRE fees for distributors ordering ≥3 units quarterly. 19 years of lean manufacturing enables flexible production scheduling.
3 Shipping Terms: Total Landed Cost Optimization
2026 shipping requires granular cost analysis beyond FOB/DDP labels:
| Term | 2026 Implementation Checklist | Critical Clause |
|---|---|---|
| FOB Shanghai | • Confirm exact loading port (Waigaoqiao vs. Yangshan) • Demand Incoterm® 2020-compliant documentation • Pre-negotiate demurrage rates ($220/day avg. 2026) |
“FOB Vessel Name, Waigaoqiao Terminal” – prevents port congestion surcharges |
| DDP Destination | • Require HS code-specific duty calculation • Verify customs bond coverage • Confirm last-mile carrier credentials • Demand real-time shipment tracking API integration |
“DDP Incoterms® 2020 + ISF filing by seller” – avoids $5,000 US customs penalties |
| Hybrid Model | FOB for manufacturing hub + DDP for final mile. Ideal for EU distributors: Shanghai → Rotterdam FOB, then DDP to Berlin. | Specify “Seller manages EU customs clearance under Article 78 MDR” |
Shanghai Carejoy Logistics: DDP-compliant shipping to 87 countries with all duties/taxes pre-paid. Baoshan District factory (20km from Waigaoqiao Port) enables 48-hour dispatch. Real-time container tracking via Carejoy Portal™.
Trusted 2026 Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Compliance Matters: Full vertical integration (chairs, CBCT, scanners) with ISO 13485-certified production lines. Direct factory pricing for dental clinics & distributors with no trading company markup.
Core Capabilities:
• Dental Chairs: 5,000+ units/month capacity
• OEM/ODM: UI customization, multi-language support
• Regulatory: CE MDR, FDA 510(k), CFDA Class III certified
• Warranty: 36-month comprehensive coverage
Contact for Verified Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: 1288 Jixi Road, Baoshan District, Shanghai 200949, China
Note: All 2026 certifications subject to verification via Carejoy’s secure supplier portal. Distributor agreements require proof of medical device license.
Frequently Asked Questions
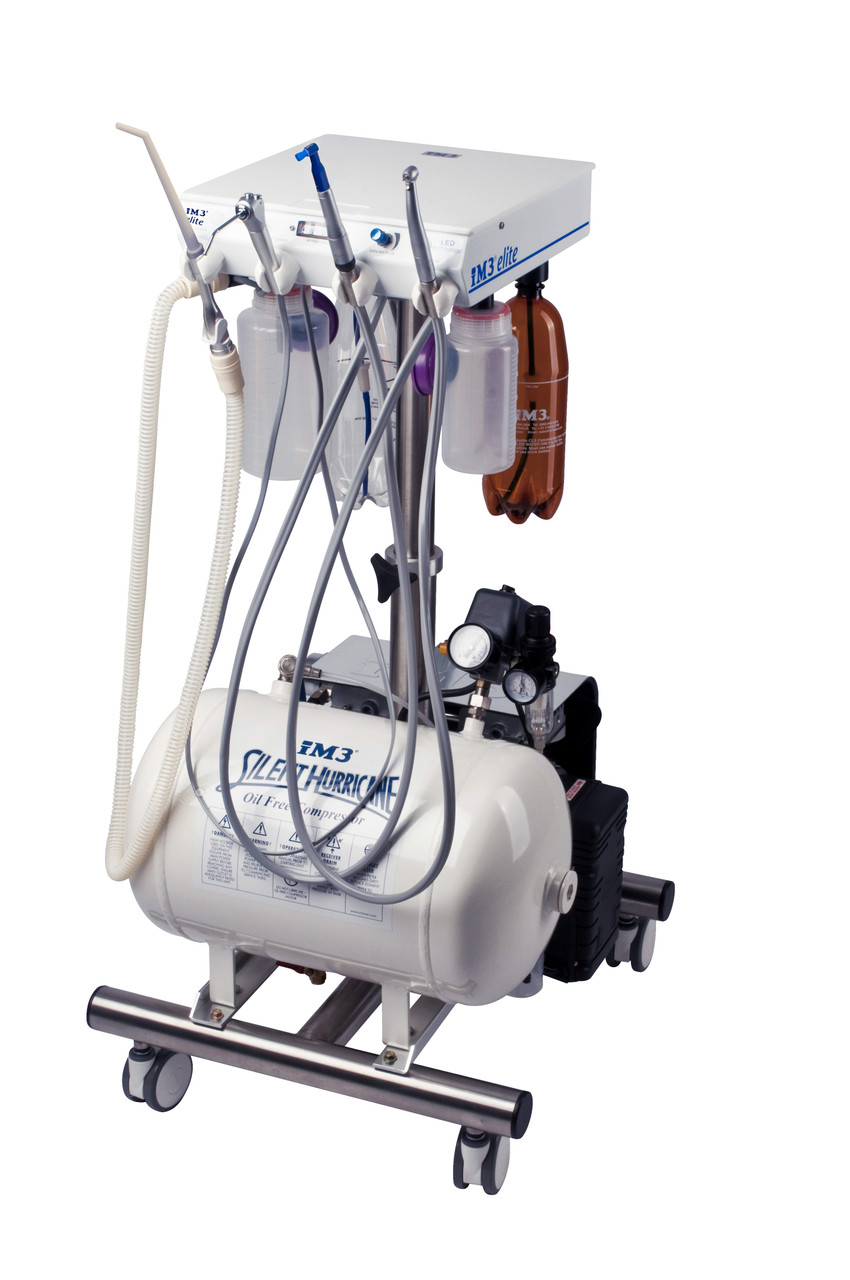
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Dental Units in 2026
As dental technology evolves and global supply chains adapt, selecting the right dental unit manufacturer requires due diligence. Below are five critical FAQs for dental clinics and distributors evaluating dental unit suppliers in 2026.
| Question | Professional Guidance |
|---|---|
| 1. What voltage compatibility should I verify when purchasing a dental unit for international or regional deployment in 2026? | Dental units must be compatible with local electrical standards. In 2026, confirm whether the unit supports 110–120V (common in North America) or 220–240V (standard in Europe, Asia, and Africa). Opt for manufacturers offering dual-voltage models or region-specific configurations. Ensure compliance with IEC 60601-1 for medical electrical safety. Units with built-in voltage regulators or auto-switching capability are recommended for multi-location clinics or distributors serving diverse markets. |
| 2. How do I ensure long-term availability of spare parts for the dental unit I purchase? | Partner with manufacturers who guarantee spare parts availability for a minimum of 10–15 years post-discontinuation of a model. Request a written parts lifecycle policy and verify the existence of regional distribution hubs. In 2026, leading manufacturers are adopting digital part catalogs and AI-driven inventory forecasting—ensure your supplier provides these tools. Distributors should confirm consignment stock options and lead times for critical components (e.g., handpiece connectors, control boards, and tubing). |
| 3. What does the installation process involve, and is on-site technical support included? | Installation of modern dental units in 2026 often includes integration with digital workflows (e.g., CAD/CAM, imaging systems). Confirm whether the manufacturer provides certified technicians for on-site setup, plumbing/electrical connections, and calibration. Turnkey installation packages should include system diagnostics, staff training, and network configuration. For distributors, ensure your supplier offers train-the-trainer programs and remote support via AR-assisted tools for faster deployment across client sites. |
| 4. What warranty terms are standard for dental units in 2026, and what do they cover? | Leading manufacturers now offer 3–5 year comprehensive warranties covering structural components, electronics, and embedded software. In 2026, expect coverage to include touchscreens, digital sensors, and connectivity modules. Verify whether labor, on-site service, and software updates are included. Extended warranty options up to 7 years are increasingly available. Distributors should confirm global warranty enforceability and service network density in their target markets. |
| 5. How are software updates and electronic component obsolescence managed under warranty and beyond? | With dental units becoming IoT-enabled platforms, manufacturers must provide scheduled software updates and cybersecurity patches for at least 7 years. Inquire about over-the-air (OTA) update capabilities and backward compatibility with future accessories. Warranty policies should clarify handling of obsolete electronic modules—prefer suppliers with modular design principles allowing component-level upgrades without full unit replacement. This is critical for future-proofing clinic investments and maintaining distributor competitiveness. |
Need a Quote for Dental Unit Manufacturer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


