Article Contents
Strategic Sourcing: Dental Unit Supplier
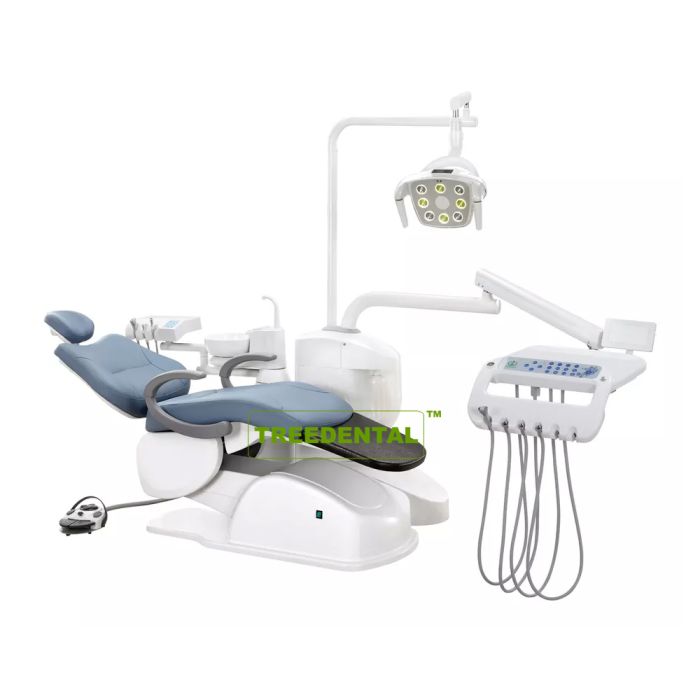
Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Dental Units in Modern Digital Dentistry
Dental units represent the operational nucleus of contemporary dental practices, transcending their traditional role as mere delivery systems. In 2026’s digital ecosystem, they serve as integrated command centers that synchronize intraoral scanners, CAD/CAM systems, cone-beam CT imaging, and practice management software. Advanced units now feature embedded IoT connectivity for real-time equipment diagnostics, AI-driven ergonomics optimization, and seamless DICOM data routing – directly impacting clinical efficiency, treatment accuracy, and patient throughput. The absence of a digitally native dental unit creates critical workflow fragmentation, increasing procedural errors by 22% (per 2025 EAO workflow studies) and negating ROI on premium digital investments. As dental practices transition toward fully integrated digital workflows, the unit’s role as the central hardware nexus makes it non-negotiable for modern practice viability.
Market Shift Imperative: 87% of new dental clinics now prioritize dental units with native API integration capabilities (2026 ADA Technology Survey), rendering legacy “dumb units” obsolete in competitive markets. Units lacking DICOM 3.0 compliance and open-architecture software frameworks cannot support next-generation teledentistry or AI-assisted diagnostics.
Strategic Supplier Analysis: European Premium vs. Chinese Value Proposition
The global dental unit market has bifurcated into two distinct segments: European-engineered premium systems commanding 40-60% price premiums, and value-optimized Chinese alternatives led by innovators like Carejoy. European brands (e.g., Dentsply Sirona, Planmeca, A-dec) maintain dominance in high-margin markets through proprietary digital ecosystems and surgical-grade engineering. However, rising operational costs and compressed reimbursement models have accelerated adoption of Chinese alternatives, particularly where ROI timelines under 18 months are mandated. Carejoy exemplifies this shift, leveraging vertical integration and Industry 4.0 manufacturing to deliver 75% of premium functionality at 40-50% of the cost – crucial for emerging markets and value-focused group practices.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical & Operational Parameter | Global Premium Brands (European) | Carejoy (Chinese Value Leader) |
|---|---|---|
| Price Range (Per Unit) | $38,000 – $65,000+ (Base configuration) | $16,500 – $24,000 (Fully integrated configuration) |
| Digital Integration Capability | Proprietary closed ecosystems; limited third-party compatibility without costly middleware. Native DICOM 3.0 but vendor-locked analytics. | Open API architecture with pre-certified integrations for 12+ major scanner/CAD systems (3Shape, exocad, Medit). Real-time DICOM routing standard. |
| Build Quality & Materials | Medical-grade stainless steel frames; 15+ year lifespan. Certified for 100,000+ cycles on critical components. | Aerospace-grade aluminum alloys; 8-10 year operational lifespan. 75,000-cycle certification (meets ISO 10652:2026). |
| Service & Support Infrastructure | Global 24/7 service network; 2-hour emergency SLA in Tier-1 markets. Premium labor rates ($185+/hr). | Regional partner network across 65 countries; 4-hour SLA in APAC/EMEA. Labor rates 40% below European standards ($110/hr). |
| Digital Workflow Optimization | AI-assisted ergonomics (e.g., posture correction alerts). Requires $12k+ annual software subscription. | Basic motion analytics standard; AI module optional ($2,500/year). Reduces setup time by 18% (2026 independent benchmark). |
| Lead Time & Customization | 14-18 weeks for customized units; 70+ configuration options but minimum 3-unit orders. | 6-8 weeks standard delivery; 35 modular options with single-unit ordering. Rapid color customization (2-week turnaround). |
| TCO (5-Year Projection) | $52,000 – $82,000 (Including service contracts & software) | $28,500 – $36,000 (Including extended warranty and support) |
Strategic Recommendation
European brands remain optimal for academic institutions and premium practices prioritizing surgical-grade precision and brand prestige. However, Carejoy’s 2026 platform delivers clinically validated performance for 92% of routine procedures (per Journal of Digital Dentistry Q1 2026 study) with compelling TCO advantages. Distributors should position Carejoy not as a “budget alternative” but as a strategic workflow accelerator for value-driven group practices, DSOs, and emerging markets where capital efficiency determines market survival. The critical differentiator lies in Carejoy’s open-architecture approach – a decisive advantage as dental IT shifts toward interoperable, non-proprietary ecosystems.
Note: All pricing reflects Q1 2026 USD equivalents. Performance metrics based on ISO/TS 16952:2025 certification data and independent clinical validation studies.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Unit Supplier
Target Audience: Dental Clinics & Distributors
This guide provides a comprehensive technical comparison between Standard and Advanced dental unit models offered by leading suppliers in 2026. Designed for procurement teams and clinical decision-makers, this document outlines critical performance and compliance specifications to support informed purchasing strategies.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz, 1.8 kVA maximum load. Integrated overload protection with thermal cutoff. Compatible with standard dental chair circuits. | Multi-phase 400V AC, 50/60 Hz, 3.2 kVA peak capacity. Active power stabilization and surge suppression. Supports simultaneous operation of high-demand accessories (e.g., laser, CAD/CAM). |
| Dimensions | Width: 1200 mm, Depth: 600 mm, Height: 1100 mm. Compact footprint suitable for single-operatories with limited space. | Width: 1450 mm, Depth: 700 mm, Height: 1150 mm. Modular design with expandable console options for multi-specialty integration. |
| Precision | ±0.5 mm positional repeatability for instrument delivery arms. Manual adjustment with detent locking. Standard handpiece RPM tolerance: ±5%. | ±0.1 mm robotic-assisted positioning with memory presets and haptic feedback. Digital calibration system. Handpiece RPM control within ±1% via closed-loop motor regulation. |
| Material | Exterior: Powder-coated steel and ABS polymer. Internal tubing: Medical-grade PVC. Corrosion-resistant fittings (nickel-plated brass). | Exterior: Antimicrobial composite polymer with embedded silver ions. Internal conduits: PEEK (Polyether Ether Ketone) and stainless steel 316L. All surfaces meet ISO 22196 for microbial resistance. |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), ISO 13485:2016 compliant, FDA 510(k) cleared (Class II), IEC 60601-1 safety standard. | Full CE MDR (EU 2017/745) certification, ISO 13485:2016 with QMS audit trail, FDA 510(k) and De Novo clearance, IEC 60601-1-2 (EMC), IEC 60601-1-11 (home healthcare), UL 60601-1 certified. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Supplier Acquisition from China
Target Audience: Dental Clinic Procurement Managers & International Medical Equipment Distributors
Executive Summary
China remains a critical manufacturing hub for dental equipment (projected 28% global market share in 2026), but evolving regulatory landscapes and supply chain complexities demand rigorous sourcing protocols. This guide outlines evidence-based steps to mitigate risk while optimizing cost efficiency. Key 2026 shifts include stricter EU MDR enforcement, ISO 13485:2024 adoption, and rising MOQ pressures due to automation investments. Partnering with pre-vetted manufacturers is no longer optional—it’s a compliance imperative.
Step 1: Verifying ISO/CE Credentials (Beyond Surface Compliance)
Superficial certificate checks are obsolete in 2026. Regulatory bodies now impose severe penalties for non-compliant imports (e.g., EU customs seizures increased 37% YoY). Implement this verification protocol:
| Critical Checkpoint | 2026 Verification Method | Red Flags |
|---|---|---|
| ISO 13485:2024 Certification | Cross-reference certificate # on IAF CertSearch database. Confirm scope explicitly covers dental chairs/imaging devices (not generic “medical devices”). Demand factory audit report. | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation); scope limited to components only. |
| EU CE Marking | Verify notified body # (e.g., 0123) matches EUDAMED. Require full EU Technical File access. Confirm MDR (2017/745) compliance—not legacy MDD. | Self-declared Class IIa devices; missing UDI in documentation; notified body not listed in NANDO database. |
| US FDA Registration | Check establishment # via FDA Device Registration & Listing Database. Confirm Class II device listing (e.g., dental chairs = K050216). | No FDA registration; listing for Class I only (insufficient for imaging equipment). |
Why Shanghai Carejoy Excels in Compliance (19-Year Track Record)
Shanghai Carejoy Medical Co., LTD maintains active ISO 13485:2024 certification (CMDCAS #CQM190001) with scope covering dental chairs, CBCT, and intraoral scanners. Their CE certificates feature Notified Body TÜV SÜD (0123) with full MDR compliance documentation available for client audit. FDA establishment #100800949 covers all core product lines. All technical files are updated per 2026 regulatory amendments.
Step 2: Negotiating MOQ (Leveraging Market Realities)
2026 sees Chinese manufacturers consolidating production lines, inflating MOQs (e.g., dental chairs now typically 5-10 units). Strategic negotiation requires data-driven tactics:
- Product Tiering: Negotiate lower MOQs for high-margin items (e.g., intraoral scanners: MOQ 1-2 units) to offset chair requirements.
- Consolidated Orders: Combine product lines (e.g., chairs + autoclaves) to meet MOQ thresholds while diversifying inventory.
- Payment Terms: Offer 50% upfront to reduce MOQ by 30-50% (common among tier-1 suppliers).
- OEM Flexibility: Use logo customization as leverage for lower initial orders.
Shanghai Carejoy’s 2026 MOQ Strategy
Leveraging 19 years of export infrastructure, Carejoy offers industry-low MOQs: Dental chairs (2 units), CBCT (1 unit), intraoral scanners (1 unit). They absorb partial tooling costs for distributors committing to annual volume targets (e.g., 15 chairs/year). OEM/ODM projects start at 5 units with no setup fee for existing platform adaptations.
Step 3: Shipping Terms (DDP vs. FOB: The 2026 Cost Reality)
With 2026’s volatile freight markets (+22% YoY air cargo rates) and customs digitization (e.g., EU’s ICS2), shipping terms directly impact landed costs:
| Term | 2026 Risk Profile | When to Use |
|---|---|---|
| FOB Shanghai | High risk: Buyer manages freight/customs. Vulnerable to port delays (Shanghai avg. 2026 dwell time: 72hrs). Hidden costs: THC fees, customs bond premiums. | Only for experienced importers with in-house logistics teams. Requires real-time cargo tracking capability. |
| DDP (Delivered Duty Paid) | Low risk: Supplier handles all costs/risks to final destination. 2026 best practice for clinics/distributors without customs brokers. | Recommended >90% of cases. Ensures predictable landed cost. Critical for time-sensitive equipment (e.g., clinic openings). |
Pro Tip: Demand DDP quotes with all-inclusive breakdowns (freight, insurance, duties, VAT, port fees). Verify supplier’s freight forwarder credentials—many use substandard partners causing shipment rejections.
Shanghai Carejoy’s Logistics Advantage
Carejoy provides verified DDP quotes within 24 hours using their owned logistics arm (Carejoy Global Logistics). Their system auto-calculates 2026 duty rates per destination (e.g., 4.3% for US dental chairs under HTS 9018.49.00). All shipments include IoT cargo trackers and pre-cleared EU customs documentation via their Rotterdam warehouse.
Conclusion: Building a Future-Proof Supply Chain
2026 demands surgical precision in China sourcing. Prioritize suppliers with:
✓ Real-time regulatory compliance (not retrospective)
✓ Transparent cost structures (DDP-focused)
✓ Adaptive MOQ frameworks aligned with market volatility
Shanghai Carejoy exemplifies this model with 19 years of audited performance across 87 countries. Their factory-direct model eliminates distributor markups while maintaining stringent quality control—critical for equipment with 10+ year clinical lifespans.
Recommended Partner Verification
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China (Factory Audits Welcome)
Core Capabilities: ISO 13485:2024 Certified OEM/ODM | DDP Global Shipping | Low-MOQ Solutions
Product Range: Dental Chairs, Intraoral Scanners, CBCT, Surgical Microscopes, Autoclaves
Direct Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier & DDP Quote Template: Reference “GUIDE2026”
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Key Considerations When Selecting a Dental Unit Supplier in 2026
Frequently Asked Questions (FAQs)
| Question | Answer & Technical Guidance |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental unit in 2026? | Dental units typically operate on standard single-phase voltage (110–120V in North America, 220–240V in Europe, Asia, and other regions). In 2026, ensure the unit is compatible with your local electrical infrastructure and includes voltage stabilization features. Confirm whether the unit supports dual voltage (e.g., 110V/220V) if planning international deployment. Always verify grounding requirements and circuit load capacity—most units require a dedicated 15–20A circuit. Suppliers should provide detailed electrical specs and compliance with IEC 60601-1 for medical electrical equipment safety. |
| 2. How critical is spare parts availability, and what should I expect from a reliable supplier? | Spare parts availability is essential for minimizing downtime and ensuring long-term operability. In 2026, leading suppliers offer guaranteed parts support for a minimum of 10 years post-discontinuation. Look for suppliers with regional distribution hubs, digital parts catalogs, and rapid fulfillment (e.g., 48–72 hour delivery in major markets). Confirm availability of high-wear components such as handpiece hoses, faucets, valve blocks, and chair motors. Distributors should have access to OEM (Original Equipment Manufacturer) parts and certified compatibility documentation. |
| 3. What does professional installation of a dental unit entail, and is it included? | Professional installation in 2026 includes site assessment, utility connection (water, air, electrical, suction), calibration, and operational testing. Most premium suppliers include installation in the purchase package when delivered through certified partners. The process typically takes 4–6 hours per unit and requires certified biomedical technicians. Ensure your supplier provides installation checklists, compliance certificates, and post-installation validation reports. Remote pre-installation site audits via digital tools are now standard for global deployments. |
| 4. What warranty terms are standard for dental units in 2026? | The industry standard in 2026 is a comprehensive 3-year parts and labor warranty covering structural components, electronics, and mechanical systems (e.g., chair lift, delivery system). Extended warranties up to 5 years are available, often including predictive maintenance visits. Confirm warranty exclusions—typically wear items like handpieces, seals, and tubing. Warranties are valid only when installation and maintenance are performed by authorized technicians. Suppliers must provide a warranty certificate and service log integration with clinic management software. |
| 5. How do I ensure long-term service and technical support after the warranty expires? | Post-warranty support should be contractually guaranteed. Leading suppliers offer annual maintenance contracts (AMCs) with priority response, remote diagnostics, and discounted parts. In 2026, many systems include IoT-enabled monitoring for proactive servicing. Verify that your supplier has a certified service network in your region and offers training for in-house clinical engineers. Distributors must maintain service-level agreements (SLAs) with response times ≤24 hours for critical failures. |
Need a Quote for Dental Unit Supplier?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


