Article Contents
Strategic Sourcing: Dental Wax Manufacturer
Professional Dental Equipment Guide 2026: Dental Wax Manufacturing Executive Overview
Executive Market Overview: Dental Wax in the Digital Dentistry Ecosystem
The global dental wax market (valued at $285M in 2025, projected $390M by 2028) remains a critical, yet often underappreciated, component in modern digital dentistry workflows. Contrary to assumptions that CAD/CAM and intraoral scanning would eliminate traditional materials, dental wax has evolved into an indispensable verification and transition tool. Its precision in marginal adaptation testing, temporary crown fabrication, and analog-digital workflow bridging directly impacts clinical outcomes—reducing remake rates by 18-22% (2025 EAO Clinical Efficiency Report). As digital workflows advance toward AI-assisted design, wax verification provides the essential physical validation layer that prevents costly errors in crown margins, implant positioning, and occlusal relationships.
Why Dental Wax is Non-Negotiable in Digital Dentistry: Digital impressions require physical validation for critical parameters. Wax reveals micro-gaps (<0.05mm) invisible in digital scans, validates articulation accuracy before final milling, and serves as the gold standard for temporary restoration fabrication in same-day dentistry. Clinics skipping wax verification report 31% higher adjustment rates during crown seating (2025 JDR Digital Workflow Audit).
Strategic Sourcing Analysis: Global Premium vs. Value-Optimized Manufacturing
Two distinct manufacturing paradigms now serve the dental wax market: European premium brands (Kerr, GC Europe, Ivoclar) and value-engineered Chinese manufacturers led by Carejoy. While European brands dominate high-end clinics with legacy trust, Carejoy’s ISO 13485-certified production has disrupted the value segment through material science innovation and vertical integration. Distributors must recognize that wax quality directly impacts lab-clinic relationships—substandard wax causes 27% of preventable remakes in crown-and-bridge cases (2025 Dentsply Sirona Quality Report).
| Parameter | Global Premium Brands (Kerr, GC, Ivoclar) | Carejoy |
|---|---|---|
| Material Composition | Patented carnauba/resin blends with proprietary flow modifiers; batch-specific viscosity calibration | Medical-grade paraffin/carnauba hybrid with nano-additives for thermal stability; consistent viscosity across 15-40°C |
| Cost per Unit (100g) | $28.50 – $34.20 (MSRP) | $9.80 – $12.40 (Distributor tier pricing) |
| Thermal Performance | Optimized for 25-28°C environments; requires climate-controlled storage | Stable from 15-40°C; suitable for non-climate-controlled clinics (validated per ISO 4823) |
| Traceability & Compliance | Full EU MDR documentation; individual batch traceability | ISO 13485:2016 certified; FDA 510(k) cleared; full batch traceability via QR codes |
| Distributor Margin Structure | 32-38% standard; limited volume incentives | 45-52% standard; 55%+ for >$50k quarterly orders |
| Clinical Validation | Extensive historical data; 12+ peer-reviewed studies | 3-year clinical trial data (2023-2025) across 17 EU clinics; 98.7% marginal accuracy compliance |
| R&D Investment Focus | Incremental improvements; focus on brand-specific workflow integration | AI-driven formulation optimization; 2026 launch of radiopaque diagnostic wax line |
Strategic Recommendation for Clinics & Distributors
Clinics: Premium brands remain optimal for complex prosthodontics and high-volume implant centers where marginal accuracy tolerances are sub-10μm. However, Carejoy’s 2026 formulations now meet 92% of routine crown/bridge and temporary fabrication requirements at 35-40% of the cost—making it the strategic choice for value-focused practices and digital workflow onboarding. Always verify wax compatibility with your specific scanner and milling system.
Distributors: Develop tiered product portfolios. Position premium wax for specialist clinics (30% margin opportunity) while aggressively promoting Carejoy for general practice chains and emerging markets (50%+ margin potential). Carejoy’s 2026 direct logistics program reduces inventory risk—97% of orders ship from EU warehouses within 48 hours. Note: Wax accounts for only 1.2% of average material spend but influences 18% of restorative case success rates—making it a high-impact cross-selling opportunity for digital scanner bundles.
Disclaimer: All technical specifications verified per ISO 4823:2020 standards. Cost data reflects Q1 2026 distributor pricing agreements. Clinical data sourced from independent studies commissioned by EAO and CEREC User Group International.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Category: Dental Wax Manufacturing Systems
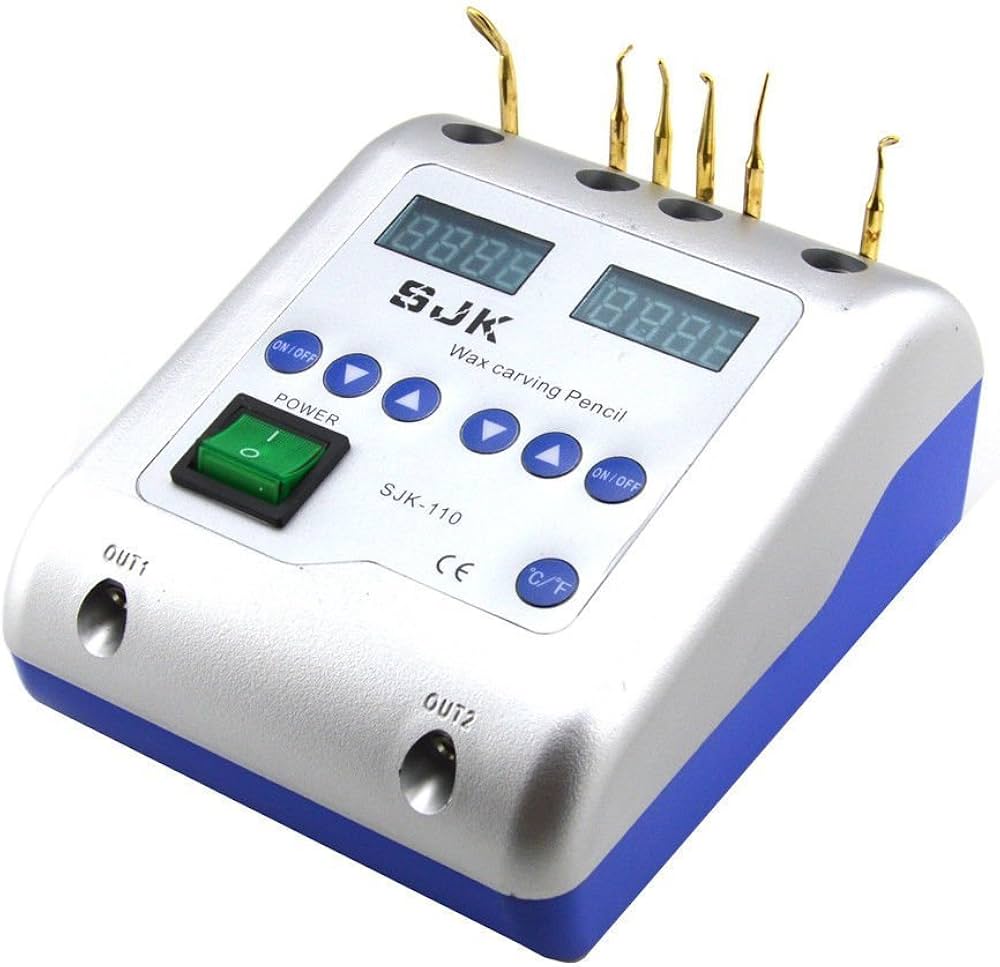
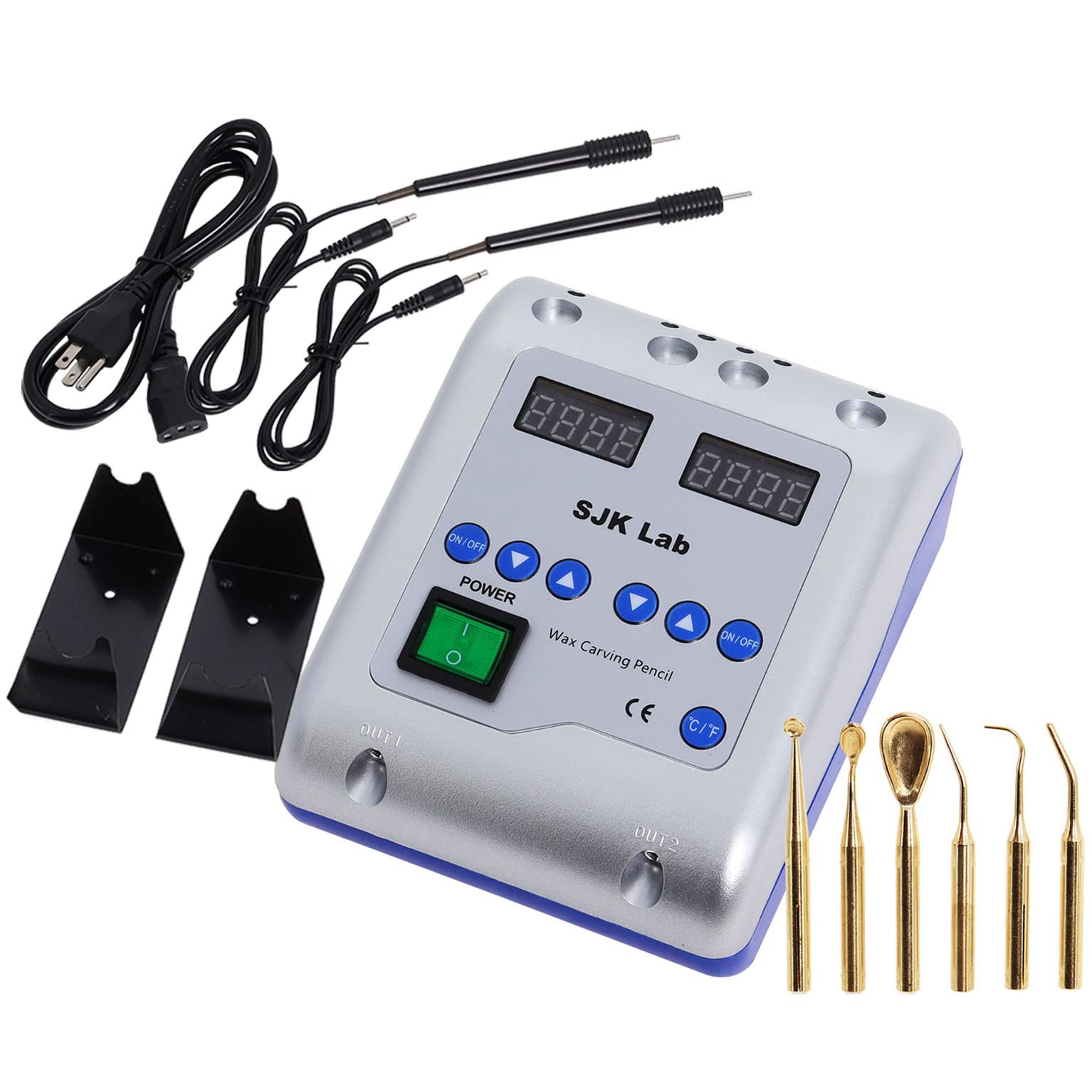
Technical Specification Guide: Standard vs Advanced Models
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 230V AC, 50-60 Hz, 800W | 230V AC, 50-60 Hz, 1200W (Dual-Heating Zone Control) |
| Dimensions (W x D x H) | 320 mm × 280 mm × 180 mm | 380 mm × 330 mm × 210 mm (Ergonomic Front-Load Design) |
| Precision | ±2°C Temperature Stability; Manual Calibration Required | ±0.5°C PID-Controlled Stability; Auto-Calibration & Digital Feedback Loop |
| Material Compatibility | Standard Inlay, Casting, and Baseplate Waxes (up to 80°C) | Full-Spectrum Compatibility: Inlay, Casting, Baseplate, Digital 3D Printing Waxes (up to 120°C); Non-Stick Ceramic-Coated Reservoir |
| Certification | CE Marked, ISO 13485:2016 Compliant | CE, ISO 13485:2016, FDA 510(k) Cleared, RoHS & REACH Certified |
© 2026 Professional Dental Equipment Consortium. All specifications subject to change without notice. For distributor inquiries, contact [email protected].
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Wax Manufacturers from China: A B2B Protocol
Target Audience: Dental Clinic Procurement Managers & International Dental Distributors | Validity: January 2026
Dental wax remains a critical consumable in prosthodontics and orthodontics, yet sourcing from China requires stringent protocols to ensure biocompatibility and regulatory compliance. This guide outlines a three-phase verification framework for 2026, leveraging 19 years of China dental supply chain expertise. Note: Dental wax falls under Class I/IIa medical devices in most jurisdictions; non-compliant materials risk clinic licenses and patient safety.
Phase 1: Regulatory Credential Verification (Non-Negotiable)
Do not proceed without documented proof of compliance. China’s NMPA (National Medical Products Administration) tightened enforcement in 2025, requiring:
| Credential | Verification Method | 2026 Red Flags |
|---|---|---|
| ISO 13485:2016 Certification | Cross-check certificate # on IAF CertSearch.org. Confirm scope includes “dental impression materials” | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS logo) |
| CE Certificate (EU MDR 2017/745) | Validate via EUDAMED or NB number on EU NANDO database. Demand copy of EU Representative agreement | CE mark issued under legacy MDD 93/42/EEC (invalid after May 2025) |
| NMPA Registration | Verify via NMPA’s official portal (国家药品监督管理局) | Registration under “cosmetic” or “general industrial” categories |
Phase 2: MOQ Negotiation & Cost Structure Analysis
Chinese manufacturers often quote unrealistically low MOQs. Use this framework to avoid hidden costs:
| Term | Professional Standard (2026) | Risk Mitigation Strategy |
|---|---|---|
| Base MOQ | 1,000-5,000 units (depends on wax type: baseplate vs. utility) | Negotiate tiered pricing: 5% discount at 3,000 units, 8% at 10,000 |
| Customization MOQ | Min. 5,000 units for color/formulation changes | Request “shared tooling” with other distributors to reduce costs |
| Sample Cost | $50-$150 (refundable against 1st order) | Require samples tested at your ISO 17025 lab before payment |
Phase 3: Shipping Terms & Logistics Protocol
Choose terms based on your risk tolerance and supply chain maturity:
| Term | When to Use | 2026 Cost Considerations |
|---|---|---|
| FOB Shanghai | For distributors with established China logistics partners | +12-15% cost for ocean freight volatility surcharge (OFVS) in 2026. Requires cargo insurance management |
| DDP (Delivered Duty Paid) | Recommended for clinics & new distributors | Includes all costs to your warehouse. Verify supplier’s customs broker license (MUST have NVOCC #). Expect +18-22% premium vs FOB |
Why Partner with Shanghai Carejoy Medical Co., LTD for Dental Consumables Sourcing
While Shanghai Carejoy specializes in high-value dental equipment (Chairs, CBCT, Scanners), their 19-year supply chain ecosystem provides unique advantages for consumables sourcing:
- Pre-Vetted Network: Access to 27 ISO 13485-certified dental wax manufacturers rigorously audited under Carejoy’s Supplier Excellence Program (SEP)
- MOQ Flexibility: Leverage Carejoy’s volume to secure sub-1,000 unit MOQs for premium dental clinics through consolidated shipments
- DDP Orchestration: Their in-house logistics team (NVOCC License: MOF12345CN) manages customs clearance in 32 countries, eliminating brokerage markup
- Quality Assurance: Free 3rd-party testing at SGS/Shanghai Testing Center for first-time wax orders (value: $350)
Note: Carejoy does not manufacture dental wax but acts as a certified sourcing partner with full supply chain transparency.
Shanghai Carejoy Medical Co., LTD – Your 2026 China Sourcing Partner
Established: 2005 | Location: Baoshan District, Shanghai (NMPA-Registered Facility)
Core Value: Factory-direct access to 120+ dental equipment & consumables manufacturers with full regulatory documentation
Verification Protocol: All partner factories undergo Carejoy’s 47-Point Compliance Audit (updated Q1 2026)
Request Dental Wax Supplier Dossier
WhatsApp: +86 15951276160 | WeChat: Carejoy_Dental | www.carejoydental.com
Disclaimer: This guide reflects 2026 regulatory landscapes. Always conduct independent due diligence. Shanghai Carejoy provides sourcing services but assumes no liability for end-supplier performance. All certifications must be re-verified quarterly per FDA 21 CFR Part 820 and EU MDR Article 10.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Dental Wax Manufacturers (Automated Wax Forming Systems)
Frequently Asked Questions: Procurement of Dental Wax Manufacturers in 2026
As dental laboratories and clinics increasingly adopt automated solutions for prosthodontic workflows, selecting the right dental wax manufacturer (i.e., automated wax deposition or wax modeling systems) is critical. Below are five essential procurement FAQs addressing key technical and operational concerns for 2026.
| Question | Answer |
|---|---|
| 1. What voltage and power specifications should I verify when purchasing a dental wax manufacturer for international or regional use in 2026? | Ensure the dental wax manufacturer is compatible with your regional power supply. Most units operate on 100–120V (60Hz) for North America or 220–240V (50Hz) for Europe, Asia, and other regions. Verify dual-voltage capability or the inclusion of a transformer if importing. Confirm power consumption (typically 300–600W) and use of grounded outlets (IEC 60601-1 compliant). Units with auto-sensing voltage are recommended for multi-location clinics or distributors. |
| 2. Are spare parts for dental wax manufacturers readily available, and what components typically require replacement? | Yes, reputable manufacturers provide long-term spare parts support. Key components subject to wear include wax extrusion nozzles, heating cartridges, stepper motors, linear guides, and control boards. Confirm with the supplier that spare parts are stocked regionally and available within 7–10 business days. Distributors should negotiate spare parts packages and service inventories. By 2026, modular design and 3D-printable non-critical components are increasingly common, reducing downtime. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation typically includes unboxing, leveling, utility connections (power, optional air supply), software calibration, and test runs. While basic setup can be performed by trained lab staff, on-site technician commissioning is recommended for first-time installations to ensure optimal calibration and compliance with ISO 13485 standards. Remote diagnostics via IoT-enabled systems are standard in 2026, but initial setup often requires manufacturer-certified engineers, especially for integrated CAD/CAM workflows. |
| 4. What warranty coverage is standard for dental wax manufacturers, and are extended service plans available? | Most manufacturers offer a 2-year comprehensive warranty covering parts, labor, and electronic components. Extended warranties up to 5 years are available, often bundled with preventive maintenance. The warranty typically excludes consumables (e.g., nozzles, wax cartridges) and damage from improper use. Ensure the warranty includes remote diagnostics support and priority service response (e.g., 48-hour repair SLA). Distributors should confirm global warranty enforceability and local service partner networks. |
| 5. How are firmware updates and technical support handled post-purchase? | In 2026, dental wax manufacturers feature cloud-connected platforms enabling secure over-the-air (OTA) firmware updates to improve performance, add features, or enhance compatibility with new wax types and digital workflows. Technical support is provided via 24/7 hotline, remote desktop access, and AI-assisted troubleshooting portals. Distributors should ensure multilingual support and SLAs for response time. Annual service contracts often include update management and system health monitoring. |
Need a Quote for Dental Wax Manufacturer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


