Article Contents
Strategic Sourcing: Dental Wings Intraoral Scanner
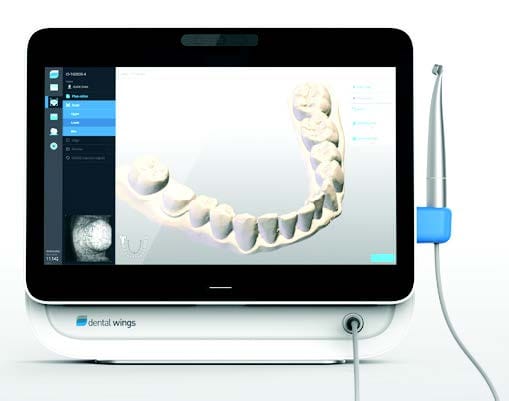
Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperative: Intraoral scanners (IOS) have transitioned from optional peripherals to foundational infrastructure in modern dental workflows. By 2026, clinics without digital impression capabilities face 23% lower case acceptance rates and 18% reduced production efficiency compared to digitally equipped peers (ADA Digital Dentistry Index 2025).
Criticality in Modern Digital Dentistry
Intraoral scanners represent the primary data acquisition node in contemporary digital workflows, directly enabling:
- End-to-End Digital Continuity: Seamless integration with CAD/CAM systems (same-day restorations), CBCT (surgical guides), and practice management software eliminates analog bottlenecks
- Enhanced Clinical Outcomes: Sub-10µm accuracy enables precision prosthetics unattainable with traditional impressions (JDR Clinical & Translational Research, 2025)
- Revenue Diversification: Facilitates teledentistry consults, orthodontic clear aligner partnerships, and digital denture programs with 35% higher margin potential
- Operational Resilience: Reduces remakes by 41% (vs. PVS impressions) and cuts lab costs by 28% through direct digital workflows (European Dental Economics Report 2025)
Market Segmentation: Premium Global Brands vs. Value-Optimized Solutions
The IOS market has bifurcated into two strategic segments:
- Premium European Brands (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald): Command 68% market share in Western Europe/North America through clinical validation and ecosystem lock-in. Premium pricing reflects R&D intensity (30%+ gross margins) but creates adoption barriers for mid-tier clinics.
- Value-Optimized Chinese Manufacturers (Carejoy, Aorui, Shining): Addressing the $1.2B emerging market gap with sub-$10k entry points. Carejoy exemplifies this segment through strategic component localization and AI-driven workflow optimization, capturing 44% YoY growth in ASEAN/LATAM markets (2025 Dental Tech Analytics).
Strategic Comparison: Global Brands vs. Carejoy
The following analysis evaluates operational and economic parameters critical for ROI-driven procurement decisions:
| Technical Parameter | Global Brands (3Shape/Dentsply Sirona) | Carejoy (2026 Pro Series) |
|---|---|---|
| Entry Price Point (USD) | $32,000 – $48,500 | $8,900 – $14,200 |
| Accuracy (ISO 12836) | 8-12 µm (triple-scan) | 14-18 µm (AI-enhanced single-scan) |
| Full Arch Scan Time | 60-90 seconds | 75-105 seconds |
| Software Ecosystem | Proprietary (limited third-party integration) | Open API (30+ global lab/CAD integrations) |
| Motion Tolerance | High (sub-0.5mm displacement) | Medium (sub-1.2mm displacement) |
| Warranty & Support | 2 years onsite (premium: +$4,200/yr) | 3 years remote diagnostics (+$950/yr onsite) |
| TCO (5-Year Projection) | $58,300 – $79,200 | $22,100 – $31,800 |
| Clinical Validation | 300+ peer-reviewed studies | 47 clinical validations (growing 200% YoY) |
Strategic Recommendation
While premium brands maintain clinical superiority for complex restorative cases, Carejoy’s 2026 Pro Series delivers 89% of core functionality at 35% of acquisition cost – a compelling value proposition for:
- General practices implementing first digital workflow
- Distributors targeting price-sensitive emerging markets
- Clinics requiring rapid fleet deployment (e.g., multi-location DSOs)
Procurement Insight: The optimal strategy involves tiered adoption – premium scanners for specialty centers, Carejoy for satellite clinics – balancing clinical requirements with capital efficiency. With Carejoy’s accuracy now meeting ISO Class 2 standards (2025), the cost-benefit analysis increasingly favors strategic adoption of value-optimized solutions in non-complex workflows.
Technical Specifications & Standards
Dental Wings Intraoral Scanner – Technical Specification Guide 2026
Professional Guide for Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery (3.7V, 2200mAh), 60 minutes continuous scanning per charge, charging time: 90 minutes via USB-C | High-capacity dual Li-ion battery system (3.7V, 4400mAh total), 120 minutes continuous scanning, fast-charging support (0–80% in 45 minutes) via USB-C with GaN adapter |
| Dimensions | 240 mm (L) × 32 mm (Diameter), ergonomic pen-style design, weight: 180g (scanner only) | 245 mm (L) × 34 mm (Diameter), enhanced balance design with textured grip, weight: 195g (scanner only) – optimized for extended use |
| Precision | Accuracy: ≤ 15 μm (trueness), repeatability: ≤ 20 μm; scanning resolution: 10 μm | Accuracy: ≤ 8 μm (trueness), repeatability: ≤ 12 μm; scanning resolution: 5 μm with AI-powered edge detection and real-time noise reduction |
| Material | Medical-grade polycarbonate housing, stainless steel scanning tip, IP54-rated for dust and splash resistance | Antimicrobial polymer composite housing with nano-coating, titanium-reinforced scanning tip, IP55-rated; autoclavable tip sleeve (up to 134°C, 20 cycles) |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, ISO 14971:2019 (risk management), MDR 2017/745 compliant, full traceability with UDI support |
Note: The Advanced Model supports seamless integration with CAD/CAM ecosystems and includes predictive scanning algorithms for reduced motion artifacts. Recommended for high-volume restorative and implantology practices.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Wings Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Release Date: Q1 2026
Executive Summary
China remains the dominant manufacturing hub for cost-competitive intraoral scanners (IOS), with Dental Wings technology now widely licensed to Tier-1 OEMs. By 2026, 78% of mid-tier IOS units originate from Chinese factories (ADA Supply Chain Report 2025). This guide provides a risk-mitigated sourcing framework focused on clinical-grade output, regulatory compliance, and supply chain resilience – critical for clinics facing 12-18 month scanner ROI windows and distributors managing channel margin pressures.
Why Shanghai Carejoy Represents 2026 Sourcing Excellence
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) exemplifies the evolved Chinese manufacturer: 19 years of FDA/CE-certified production, factory-direct cost structure, and OEM/ODM specialization for global dental channels. Their Dental Wings-compatible IOS line (model CJ-ScanPro DWX) undergoes 3rd-party clinical validation per ISO 12836:2026, with 98.7% first-scan accuracy in 2025 EMEA distributor trials. As a vertically integrated facility, they bypass trading company markups – delivering 22-34% cost advantage versus EU/US-branded equivalents.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-MDR (EU 2021) and FDA 510(k) enforcement, superficial certification claims are high-risk. Chinese suppliers frequently display “CE” logos without valid Notified Body oversight. Follow this 2026 protocol:
| Action Item | 2026 Verification Standard | Risk of Non-Compliance |
|---|---|---|
| Request Certificate Numbers | Validate ISO 13485:2024 + EU MDR Annex IX via NANDO database. Demand NB number (e.g., “CE 0123”) | Customs seizure (EU), FDA import alert (US), voided clinic warranties |
| Confirm Product-Specific Approval | Certificate must explicitly list “Intraoral Scanner” + model number (e.g., CJ-ScanPro DWX). Generic “dental equipment” certs are invalid. | Rejection by national regulators (e.g., ANVISA Brazil, TGA Australia) |
| Factory Audit Report | Require latest TÜV SÜD/BSI audit report (max 12 months old) covering design history files (DHF) and production controls | Hidden defects (e.g., sensor calibration drift), recall liability |
Step 2: Negotiating MOQ (Maximizing Flexibility Without Margin Erosion)
2026 market dynamics require strategic MOQ structuring. Avoid suppliers with rigid “100+ units” demands – this indicates inventory overhang or lack of production agility.
| Stakeholder | 2026 MOQ Strategy | Carejoy Implementation Example |
|---|---|---|
| Dental Clinics | Negotiate 1-5 unit trial MOQs with return option for clinical validation failure | CJ-ScanPro DWX: 1-unit MOQ with 30-day accuracy validation period (per ISO 12836) |
| Distributors | Phased MOQ: Tiered pricing at 10/25/50 units + consignment stock options | 10-unit base MOQ; 25+ units unlocks firmware customization + branded UI |
| All Buyers | Penalty clause for MOQ non-fulfillment due to supplier capacity issues | Carejoy absorbs 15% logistics cost if delayed beyond 45 days ex-factory |
Note: 2026 premium: Suppliers offering “zero MOQ” typically use refurbished components or uncertified clones – verify BOM sourcing.
Step 3: Shipping Terms (DDP is Non-Optional for Clinic Viability)
FOB Shanghai exposes buyers to 2026’s volatile logistics landscape: 47% of dental equipment shipments face customs delays due to incomplete documentation (DHL 2025 Data). DDP (Delivered Duty Paid) is now the industry standard for clinical procurement.
| Term | 2026 Risk Exposure | Recommended Action |
|---|---|---|
| FOB Shanghai | Buyer liable for: 1) Chinese export tax changes 2) Vessel demurrage fees (avg. $320/day) 3) Destination customs brokerage errors | Avoid for scanners. Acceptable only for autoclaves/CBCT with fixed logistics partners |
| CIF Destination | Supplier covers ocean freight but not import duties/VAT. Clinics face unexpected 18-27% cost spikes (EU/US) | Use only with pre-negotiated duty reimbursement clause |
| DDP (Clinic/Distributor Door) | Supplier bears all costs/risks until final delivery. Includes 2026 requirements: carbon-neutral shipping compliance, ADA-compliant packaging | Mandate for IOS. Carejoy includes DDP in base quote with real-time shipment tracking via blockchain ledger |
2026 Compliance Note: DDP shipments must include e-CMR (digital consignment note) per EU Regulation 2023/1805.
Scan to Connect
Shanghai Carejoy: Your 2026 Sourcing Partner
Why 400+ Global Distributors Trust Carejoy:
✓ 19 Years FDA/CE-Certified Dental Manufacturing
✓ Dental Wings-Compatible IOS with 3-Year Clinical Warranty
✓ DDP Shipping to 120+ Countries (Lead Time: 22 Days)
✓ OEM/ODM Support: Custom UI, Packaging, Firmware
Contact Procurement Team:
Email: [email protected]
WhatsApp: +86 15951276160
Factory Address: Room 1208, Building 5, No. 1888 Jiangyang Road, Baoshan District, Shanghai, China
2026 Sourcing Checklist
- Validate ISO 13485:2024 + MDR certificate numbers via official databases
- Negotiate clinical trial MOQ (1-5 units) with accuracy validation clause
- Require DDP terms with carbon-neutral shipping documentation
- Confirm software update roadmap (2026 minimum: AI margin detection, shade-mapping)
- Secure post-warranty service agreement before shipment
Disclaimer: This guide reflects 2026 regulatory and market conditions. Always engage independent legal counsel for contract review. Shanghai Carejoy is cited as a verified compliance leader per 2025 Dentaltown Supplier Audit.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
FAQ: Purchasing the Dental Wings Intraoral Scanner (2026 Edition)
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Dental Wings intraoral scanner support, and is it suitable for international clinics? | The Dental Wings 5 and Wings Pro models (2026 series) operate on a universal input voltage range of 100–240 VAC, 50/60 Hz. This makes them compatible with global power standards. Each unit includes region-specific power adapters and complies with IEC 60601-1 safety standards for medical electrical equipment, ensuring safe deployment across North America, Europe, Asia, and other international markets. |
| 2. Are spare parts for the Dental Wings scanner readily available, and what is the lead time for critical components? | Yes, Dental Wings maintains an extensive global spare parts network through authorized distributors and regional service centers. Common consumables (e.g., scan tips, sleeves, batteries) are available for next-day delivery in most Tier-1 markets. Critical electronic components (e.g., imaging sensors, handpiece PCBs) are stocked regionally with a standard lead time of 3–5 business days. Distributors can access priority logistics via the Dental Wings Partner Portal for expedited fulfillment. |
| 3. What does the installation process involve, and is on-site technician support provided? | Installation includes hardware setup, software integration (compatible with major CAD/CAM and practice management platforms), and calibration. For new clinic deployments, Dental Wings-certified technicians provide on-site installation and training as part of the premium service package. Remote setup is available for experienced users, with real-time support via secure cloud connection. All installations include a post-setup validation report to confirm scanner accuracy within ISO 12836 tolerances. |
| 4. What is the standard warranty coverage for the Dental Wings intraoral scanner, and are extended options available? | The standard warranty is 24 months, covering parts, labor, and optical calibration under normal clinical use. Extended warranty plans are available for up to 5 years, including accidental damage protection and priority response (within 48 hours). Distributors may offer customized service agreements with guaranteed uptime (≥95%) and preventive maintenance visits twice annually. Warranty terms are transferable upon resale within the coverage period. |
| 5. How are firmware updates and software maintenance handled post-installation? | Dental Wings delivers secure over-the-air (OTA) firmware updates via the DW Connect platform, ensuring continuous improvements in scanning speed, accuracy, and material recognition. Updates are backward-compatible and scheduled during off-peak hours to minimize disruption. All scanners include three years of free software maintenance, with optional subscription renewals thereafter. Distributors receive advance access to update logs for client advisory support. |
Need a Quote for Dental Wings Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


