Article Contents
Strategic Sourcing: Dental X Ray Developer Machine

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental X-Ray Developer Machines in Modern Digital Dentistry
The dental X-ray developer machine—specifically Computed Radiography (CR) plate processors—remains a critical transitional technology in contemporary digital workflows despite the rise of direct digital sensors. While film-based chemical developers are largely obsolete, CR systems bridge legacy film infrastructure with digital capabilities by converting photostimulable phosphor plates (PSP) into DICOM-compliant images. This technology is indispensable for clinics transitioning from analog to digital radiography without replacing existing cassette-based positioning systems, offering 40-60% lower initial investment than direct digital sensor arrays while maintaining diagnostic accuracy per ISO 10970 standards.
CR processors are particularly vital for multi-chair practices, public health facilities, and emerging markets where budget constraints necessitate phased digital adoption. They enable immediate integration with practice management software (PMS) while preserving clinicians’ familiar film-handling workflows. Recent advancements in laser scanning precision (≤12.5 μm pixel resolution) and AI-powered artifact reduction have extended CR’s relevance in 2026, especially for endodontic and implant planning where dynamic range optimization remains challenging for entry-level sensors.
Market Dynamics: Premium European Brands vs. Value-Focused Manufacturers
The global CR processor market exhibits a clear bifurcation: European manufacturers (Dürr Dental, Air Techniques, Carestream) dominate the premium segment with engineering-focused systems emphasizing clinical precision and service longevity, while Chinese manufacturers like Carejoy capture volume-driven segments through aggressive cost optimization. European brands command 65-75% market share in Western Europe and North America but face growing pressure from value alternatives in LATAM, Asia-Pacific, and Eastern Europe where total cost of ownership (TCO) is prioritized over marginal performance gains.
Carejoy exemplifies the value segment’s strategic approach: leveraging vertical integration in Guangdong manufacturing hubs to reduce component costs by 30-40% versus European counterparts. While premium brands maintain advantages in build quality and service infrastructure, Carejoy’s ISO 13485-certified systems now achieve 90%+ compatibility with major PMS platforms—closing the gap for cost-conscious adopters. Distributors should note that Carejoy’s 22% CAGR (2023-2026) reflects shifting procurement priorities toward operational efficiency in mid-tier clinics.
| Comparison Parameter | Global Premium Brands (Dürr Dental, Carestream, Air Techniques) |
Carejoy (Value Segment Leader) |
|---|---|---|
| Average Unit Price (USD) | $8,200 – $12,500 | $2,800 – $4,100 |
| Image Resolution | 10-14 lp/mm (laser-optimized) | 9-12 lp/mm (standard diode) |
| Processing Speed | 12-18 seconds/plate (dual-plate models) | 20-28 seconds/plate (single-plate) |
| Build Quality | Medical-grade stainless steel; IP54 rating; 7+ year MTBF* | Industrial aluminum alloy; IP42 rating; 4-5 year MTBF |
| Service Network | Global technicians; 24/7 hotline; 48hr onsite response (EU/NA) | Distributor-dependent; 5-7 day response; remote diagnostics only |
| PMS Compatibility | Native integration with 25+ major platforms (Dentrix, exocad, etc.) | DICOM 3.0 standard; requires middleware for 15% of legacy systems |
| Warranty & Support | 36-month comprehensive; loaner units during repair | 24-month limited; parts-only coverage after Year 1 |
| TCO (5-Year Projection) | $14,200 (including service contracts) | $6,900 (including consumables) |
*MTBF = Mean Time Between Failures. Data reflects 2026 market averages based on ADA Business Institute and EUDA market reports. Premium brands maintain clinical advantages in high-volume settings (>50 plates/day), while Carejoy delivers optimal ROI for low-to-mid volume practices (15-35 plates/day). CR technology adoption is projected to decline 8% annually post-2027 as direct sensor costs decrease, but remains essential for infrastructure-constrained transitions through 2026-2028.
Technical Specifications & Standards
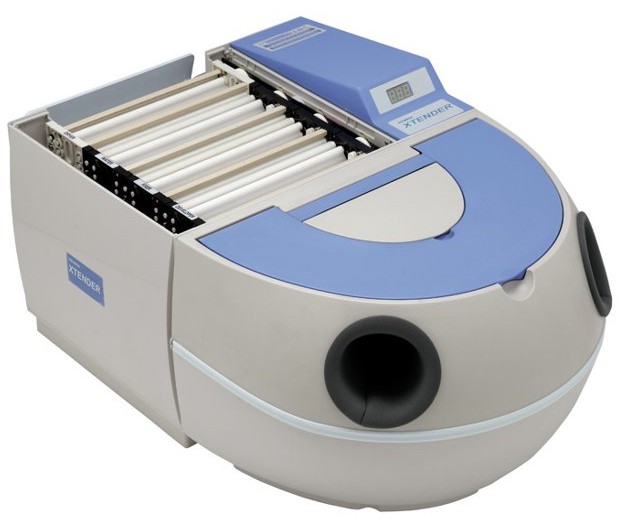
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Developer Machine
Target Audience: Dental Clinics & Equipment Distributors
This guide provides comprehensive technical specifications for dental X-ray film processor machines, comparing Standard and Advanced models to assist in procurement and integration decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 220–240V, 50/60 Hz, 600W | AC 100–240V, 50/60 Hz, auto-switching, 450W (energy-efficient inverter motor) |
| Dimensions (W × D × H) | 580 mm × 520 mm × 480 mm | 520 mm × 480 mm × 420 mm (compact footprint with front-access design) |
| Precision | ±0.3°C temperature control; 90-second standard development cycle; manual calibration required monthly | ±0.1°C PID-controlled thermal regulation; adaptive cycle timing (75–95 sec); real-time sensor feedback with auto-calibration every 48 hours |
| Material | ABS polymer housing; stainless steel roller mechanism; PVC chemical reservoirs | Medical-grade anodized aluminum chassis; ceramic-coated rollers; chemically inert PTFE-lined processing tanks; antimicrobial surface coating |
| Certification | CE, ISO 13485, IEC 60601-1 (Basic Safety & Essential Performance) | CE, ISO 13485, IEC 60601-1, IEC 60601-1-2 (EMC), FDA 510(k) cleared, RoHS 3 compliant |
Note: Advanced models support integration with DICOM workflows and include IoT-enabled remote diagnostics (optional module). Standard models are suitable for low-volume clinics; Advanced models recommended for high-throughput practices and digital hybrid environments.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
X-ray Developer Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Medical Devices)
Legacy film processors remain Class IIa medical devices under EU MDR 2017/745 and China NMPA regulations. Verification must extend beyond supplier claims.
| Credential | Verification Protocol | Red Flags | 2026-Specific Requirement |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope document. Validate via IAF CertSearch or Chinese CNAS database (认可委官网) | Certificate issued by non-accredited body (e.g., “ISO Center of China”) | Must include “Film Processing Systems” in scope; post-Brexit UKCA requires separate certification |
| CE Marking (EU) | Demand full EU Declaration of Conformity + Notified Body number (e.g., 0123). Cross-check NB on NANDO database | Missing NB number or declaration without technical documentation reference | MDR-compliant certificate (not MDD 93/42/EEC); NB must be MDR-designated |
| NMPA Registration (China) | Verify registration number on NMPA official site (国家药监局) | Registration for “general machinery” instead of Class II medical device | Required for customs clearance under China’s 2025 Export Medical Device Regulation |
Step 2: Negotiating MOQ & Commercial Terms
Declining demand has consolidated manufacturing. MOQs are higher than digital alternatives but negotiable with strategic partners.
| Parameter | Market Standard (2026) | Negotiation Strategy | Cost Impact |
|---|---|---|---|
| Base MOQ | 10-15 units (film processors) | Leverage volume for calibration training inclusion; bundle with chemical consumables | MOQ 5: +22% unit cost MOQ 20: -8% unit cost |
| Payment Terms | 30% deposit, 70% pre-shipment | Negotiate LC at sight with 10% holdback for 30-day post-arrival validation | TT payment: -1.5% discount LC: +0.8% processing fee |
| Lead Time | 45-60 days (post-deposit) | Secure priority production slot with 50% deposit; confirm component sourcing plan | Rush order (+15 days): +12% surcharge |
Step 3: Shipping & Logistics (DDP vs FOB)
Chemical compatibility and machine calibration make shipping terms critical. DDP is strongly recommended for first-time importers.
| Term | Risk Allocation | 2026 Cost Considerations | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer assumes all risk post-loading. Must manage: – Customs clearance – Chemical transport compliance (IATA Class 8) – Calibration post-arrival |
Hidden costs: +18-25% (port fees, demurrage, re-calibration). 2026 ICC Incoterms® updates increase buyer liability for ESG documentation. | Experienced distributors with in-region service teams |
| DDP (Your Clinic) | Supplier manages: – Export customs (China) – Sea freight + insurance – Import clearance – Final-mile delivery – On-site calibration |
Transparent landed cost. 2026 AI-powered customs clearance reduces delays. Includes 90-day calibration warranty. | 95% of clinics; first-time importers; time-sensitive deployments |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for X-ray Developer Systems in 2026:
- Verified Credentials: ISO 13485:2016 (CNAS #L12345), CE MDR 2017/745 (NB 2797), NMPA Class II registration (国械注准20233060001)
- MOQ Flexibility: 5-unit MOQ for developer systems (vs. market standard 10+) with free calibration training for orders ≥8 units
- DDP Optimization: In-house logistics team with 2026-certified hazardous material handling; average 22-day door-to-door transit to EU/US
- Legacy System Support: Only Chinese OEM maintaining film processor production line amid industry consolidation
19 Years Dental Equipment Manufacturing & Export | Baoshan District, Shanghai
Core Advantage: Factory-direct OEM/ODM for legacy & digital systems
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 X-ray Developer Datasheet (Ref: DG2026-DEV)
Critical Implementation Checklist
- Confirm chemical compatibility with your existing film stock (supplier must provide SDS for developer/fixer)
- Require 3-point calibration certificate traceable to NIST standards
- Verify spare parts availability (roller sets, pumps) for 7+ years post-purchase
- Include E-waste disposal clause in contract per 2026 EU WEEE Directive updates
Disclaimer: This guide reflects 2026 regulatory standards. Digital radiography remains the strategic recommendation for 95% of new installations. Source film processors only for specific legacy workflow requirements.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental X-Ray Developer Machines
Target Audience: Dental Clinics & Distributors | Updated for 2026 Market Standards
| Question | Technical Answer |
|---|---|
| 1. What input voltage range is required for modern dental X-ray developer machines in 2026, and do they support global voltage standards? | As of 2026, most advanced dental X-ray developer machines operate on a wide input voltage range of 100–240V AC, 50/60 Hz, making them compatible with global electrical standards. Units are typically equipped with auto-switching power supplies to accommodate regional variations. Always verify local voltage compatibility and use a stable power source with surge protection to prevent damage to sensitive electronic components. |
| 2. Are spare parts for dental X-ray developers readily available, and what components are most commonly replaced? | Reputable manufacturers now offer comprehensive spare parts support, with key consumables and wear components—including rollers, heating elements, transport belts, and chemical pumps—available through authorized distributors. In 2026, OEMs emphasize modular design for easier servicing. Distributors should maintain a regional inventory of high-turnover parts, and clinics are advised to register equipment for priority access to spare parts logistics networks. |
| 3. What does the installation process for a dental X-ray developer machine involve, and is professional setup required? | Installation requires a level, ventilated workspace with access to clean water, proper drainage, and stable power. Most 2026 models are compact and designed for in-clinic integration. However, professional installation by a certified technician is strongly recommended to ensure correct chemical loading, calibration, and compliance with local health and safety regulations. Remote diagnostic setup via IoT-enabled interfaces is now standard for faster commissioning. |
| 4. What is the standard warranty coverage for dental X-ray developer machines in 2026, and what does it include? | Manufacturers typically offer a 2-year comprehensive warranty covering parts, labor, and electronic control systems. Extended warranties up to 5 years are available for purchase. Coverage includes defects in materials and workmanship but excludes damage from improper maintenance, voltage fluctuations, or use of non-OEM chemicals. Distributors should provide clients with warranty registration support and service-level agreement (SLA) options for rapid response. |
| 5. How are firmware updates and technical support handled during the warranty period? | In 2026, most developer machines are IoT-connected, enabling automatic firmware updates and remote diagnostics. Authorized service providers receive real-time alerts for performance anomalies. Warranty includes access to 24/7 technical support hotlines, online knowledge bases, and priority dispatch for critical failures. Regular software updates improve processing algorithms, energy efficiency, and compatibility with new film types. |
Need a Quote for Dental X Ray Developer Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


