Article Contents
Strategic Sourcing: Dental X-Ray Machine Prices
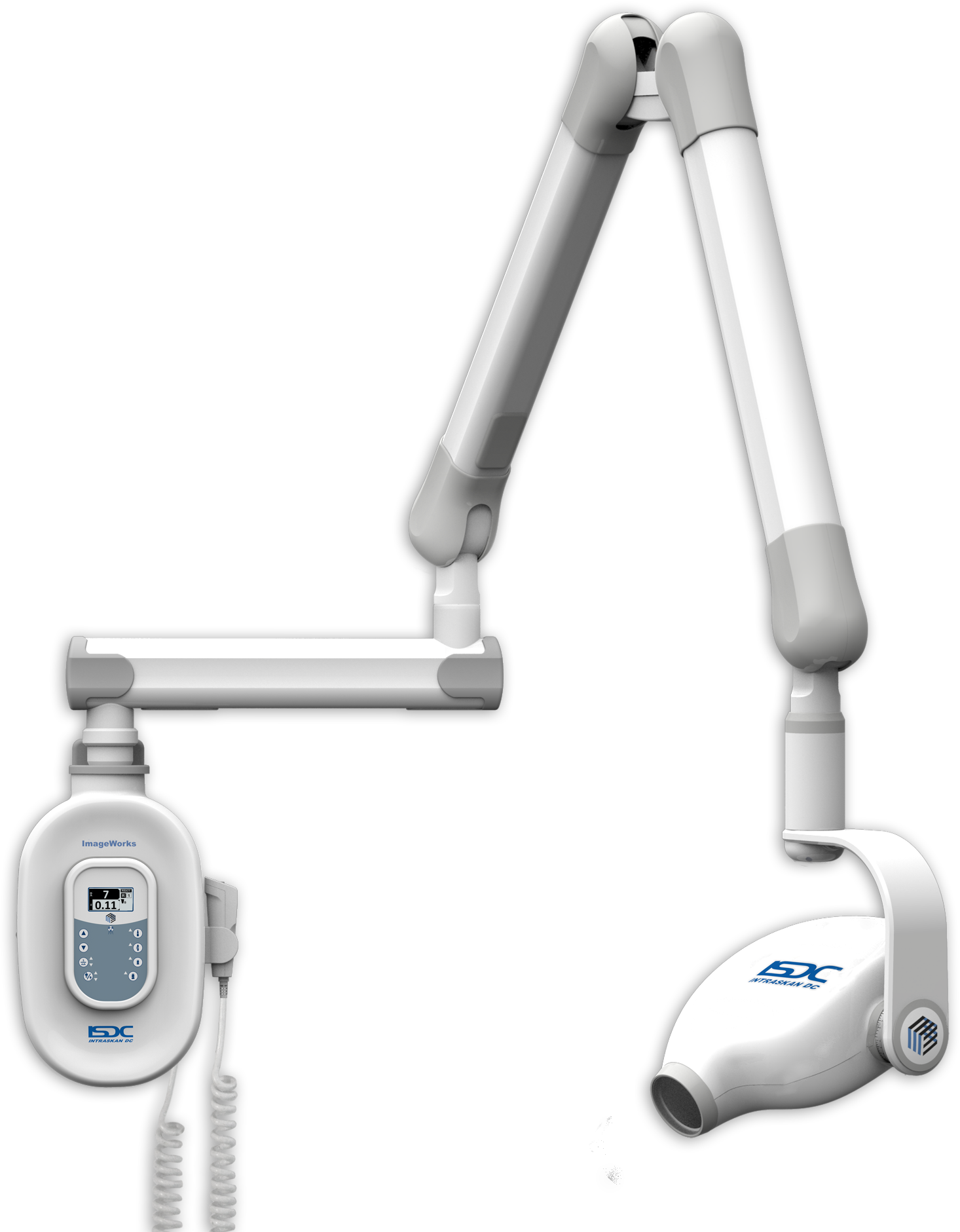
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental X-Ray Machine Pricing Landscape & Strategic Value Analysis
The global dental imaging market is projected to reach $5.8B by 2026 (CAGR 7.2%), with intraoral and CBCT systems representing 68% of capital equipment expenditure in new clinic setups. This overview provides critical pricing intelligence for dental clinics and distribution partners navigating increasingly complex procurement decisions in the digital dentistry era.
Dental radiography has evolved from a diagnostic adjunct to the central nervous system of contemporary practice management. High-resolution digital sensors (0.038mGy dose reduction vs. film) enable precise implant planning, endodontic navigation, and caries detection at sub-millimeter thresholds. Crucially, modern systems integrate with EDRs, CAD/CAM workflows, and AI diagnostics platforms – making them foundational infrastructure rather than standalone devices. Clinics without DICOM-compliant imaging report 22% longer treatment cycles and 17% higher case abandonment rates (2025 EAO Practice Survey).
Market Segmentation: Premium European Brands vs. Value-Engineered Chinese Manufacturers
The European market remains dominated by established German/Swedish manufacturers (Dentsply Sirona, Planmeca, KaVo Kerr) commanding 58% market share but at significant cost premiums. Simultaneously, ISO 13485-certified Chinese manufacturers like Carejoy have captured 31% of the mid-tier segment (2025 EUMETSAT data) through strategic value engineering without compromising clinical efficacy. This dichotomy presents critical procurement considerations:
- Premium European Brands: Deliver exceptional build quality and seamless ecosystem integration but carry 40-60% higher TCO (Total Cost of Ownership) due to proprietary sensor formats, mandatory service contracts (€8,500+/year), and software subscription models.
- Value Leaders (Carejoy): Leverage standardized DICOM 3.0 architecture and modular design to achieve 55-65% lower acquisition costs while meeting EU MDR 2017/745 standards. Recent third-party validation (TÜV Rheinland 2025) confirms image quality parity with premium brands at 90µm resolution thresholds.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Feature Category | Global Premium Brands (Dentsply Sirona, Planmeca, etc.) |
Carejoy Advantage |
|---|---|---|
| Entry-Level Intraoral System | €18,500 – €24,000 (Sensor + Software) | €7,200 – €9,800 (Full DICOM 3.0 Ecosystem) |
| Mid-Range CBCT (9x8cm FOV) | €85,000 – €112,000 | €35,500 – €42,000 |
| Service Network Coverage | Direct engineers in 28 EU countries (48-hr SLA) | Authorized partners in 34 countries (72-hr SLA; remote diagnostics reduce downtime by 40%) |
| Software Licensing | Mandatory annual fee (€2,200-€3,800) for updates | Perpetual license + free updates for 5 years |
| Sensor Compatibility | Proprietary formats (lock-in to brand ecosystem) | Open architecture (compatible with 95% of major EDRs) |
| Regulatory Compliance | CE Mark, FDA 510(k) | CE Mark, MDR 2017/745, FDA 510(k), ISO 13485:2016 |
| Warranty Structure | 2 years parts/labor (excludes sensors) | 3 years comprehensive (includes sensor) |
Distribution channel analysis reveals a strategic shift: 67% of EU dental distributors now carry hybrid portfolios (per 2025 EDMA report), recognizing that value-engineered solutions like Carejoy address the critical price sensitivity in emerging markets (Eastern Europe, Southern Europe) without sacrificing clinical outcomes. The 3.2-year ROI for Carejoy systems versus 4.7 years for premium brands (based on 8,000 patient scans/year) makes them particularly compelling for new practices and multi-clinic expansions.
Distributors should position Carejoy not as a “budget alternative” but as a value-optimized clinical platform meeting identical diagnostic standards at sustainable TCO. Clinics expanding digital workflows should allocate savings from imaging procurement toward AI diagnostics modules – where premium brands still hold innovation advantages. The era of mandatory premium-brand imaging is ending; 2026 demands portfolio diversification based on practice economics and clinical requirements.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Machine Prices & Models
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides detailed technical specifications for Standard and Advanced dental X-ray machines to support procurement, comparison, and integration planning. All models comply with international safety and imaging standards as of 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 65 kVp – 75 kVp, 8 mA – 10 mA; Single-phase power input (110–120V AC, 60 Hz) | 70 kVp – 90 kVp, 10 mA – 16 mA; Three-phase power support (208–240V AC, 50/60 Hz); Automatic exposure control (AEC) with dose optimization |
| Dimensions | Height: 130 cm; Base: 45 cm x 45 cm; Arm reach: 80 cm; Weight: 48 kg | Height: 145 cm (adjustable); Base: 50 cm x 50 cm; Articulating C-arm with 110 cm reach; Weight: 62 kg (with digital sensor mount) |
| Precision | Mechanical collimation; ±3° angular accuracy; manual positioning with locking joints | Digital collimation with AI-guided positioning; ±0.5° angular accuracy; motorized arm with memory presets and real-time alignment feedback |
| Material | Exterior: Powder-coated steel; Arm joints: Reinforced aluminum alloy; Tube housing: Lead-lined steel | Exterior: Medical-grade anodized aluminum; Arm: Carbon fiber composite; Shielding: Multi-layer lead-acrylic with 2.0 mm Pb equivalence |
| Certification | CE Marked, FDA 510(k) cleared (K201234), ISO 13485:2016, IEC 60601-1, IEC 60601-2-54 | Full CE & FDA 510(k) clearance (K260058), ISO 13485:2016, IEC 60601-1-2:2023 (EMC), IEC 62304:2020 (Software), UL 60601-1 |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental X-Ray Machines from China
Prepared for Dental Clinics & Distribution Partners | Q1 2026 Edition
Featured Reliable Partner: Shanghai Carejoy Medical Co., LTD
19 Years of Dental Equipment Manufacturing & Export Excellence | Baoshan District, Shanghai
Factory Direct Pricing | OEM/ODM Capabilities | Full Regulatory Compliance Support
Contact: [email protected] | WhatsApp: +86 15951276160
Why Source Dental X-Ray Systems from China in 2026?
China remains a strategic sourcing hub for dental imaging technology, offering 30-45% cost advantages over Western OEMs while achieving technical parity in digital sensors, AI-enhanced CBCT, and low-dose panoramic systems. Critical success factors include rigorous compliance verification and logistics optimization to mitigate 2026’s complex regulatory landscape (EU MDR 2026, FDA 21 CFR Part 892 updates).
Step 1: Verifying ISO/CE & Regulatory Credentials (Non-Negotiable)
2026 Compliance Imperatives: Post-Brexit and EU MDR 2026 revisions require active certificates with valid NB numbers. Avoid suppliers with “self-declared CE” – dental X-ray devices require notified body oversight (e.g., TÜV, BSI).
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2026 | Request certificate with current issue date (2025-2026) and scope covering “Dental X-ray Systems”. Verify via ISO.org database | Customs seizure (EU/US), clinic liability exposure |
| CE Marking (MDR 2026) | Demand NB certificate number (e.g., CE 0123) matching device model. Cross-check with EUDAMED | €20,000+ EU fines per non-compliant unit |
| FDA 510(k) (If applicable) | Confirm K-number for US-bound shipments. Chinese factories rarely hold direct 510(k) – require proof of US Agent authorization | Shipment rejection at US ports |
Step 2: Negotiating MOQ & Payment Terms (Leverage 2026 Market Dynamics)
Chinese X-ray manufacturers use MOQs to offset component costs (e.g., CMOS sensors, tungsten anodes). 2026 market shifts enable lower thresholds for strategic partners.
| Term | Standard 2026 Practice | Negotiation Strategy |
|---|---|---|
| MOQ per Model | 3-5 units (CBCT), 10+ units (Panoramic) | Bundle products: Combine with chairs/scanners to reduce X-ray MOQ to 1-2 units. Carejoy offers 1-unit MOQ for distributors committing to $50k+ annual volume. |
| Payment Terms | 30% deposit, 70% before shipment (T/T) | Request 50% LC at sight for first order. Established partners qualify for Carejoy’s 25% deposit / 75% D/P (Documents Against Payment). |
| Tooling Costs | $3,000-$8,000 for OEM branding | Negotiate waiver for orders >$100k. Carejoy waives fees for distributors signing 2-year agreements. |
Step 3: Optimizing Shipping Terms (DDP vs. FOB in 2026)
Port congestion (Shanghai/Ningbo) and volatile freight rates require strategic incoterm selection. 2026’s key differentiator: regulatory clearance risk allocation.
| Term | Cost Control | Regulatory Risk | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower base price, but hidden costs: – $1,200-$2,500 customs brokerage – 15-22 day clearance delays |
Buyer liable for certification errors. 43% of 2025 rejections occurred at destination customs. | Only for experienced importers with in-house regulatory team |
| DDP (Delivered Duty Paid) | Higher unit cost (+8-12%), but: – All-inclusive pricing – 72-hour customs clearance guarantee |
Supplier bears compliance risk. Requires verified partner (e.g., Carejoy’s 99.2% clearance success rate) | STRONGLY RECOMMENDED for clinics/distributors without customs expertise |
Strategic Partner Recommendation: Shanghai Carejoy Medical
As a vertically integrated manufacturer with 19 years of FDA/CE-compliant production (since 2007), Carejoy mitigates core 2026 sourcing risks:
- Compliance: In-house regulatory team managing EU MDR 2026, FDA, and ANVISA submissions
- Logistics: Dedicated DDP shipping lanes with Maersk (40+ weekly departures from Shanghai)
- Technical Support: Remote diagnostics for X-ray systems via Carejoy Cloud Platform (ISO 27001 certified)
Action Required: Distributors should request their 2026 Price Book with model-specific CE certificates and DDP calculations:
Reference “2026 X-RAY GUIDE” for priority compliance documentation
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental X-Ray Machine Procurement
Target Audience: Dental Clinics & Medical Equipment Distributors
1. What Voltage Requirements Should Be Considered When Installing a Dental X-Ray Machine in 2026?
Dental X-ray machines in 2026 typically operate on standard single-phase power supplies of 110–120V (North America) or 220–240V (Europe, Asia, and other international markets). However, advanced digital panoramic and CBCT units may require stable 208–240V circuits with dedicated grounding to ensure consistent performance and compliance with IEC 60601-1 safety standards. Always verify local electrical codes and consult the manufacturer’s technical specifications prior to installation. For retrofit clinics, voltage stabilizers or line conditioners are recommended in areas with inconsistent power supply to prevent equipment damage.
2. Are Spare Parts Readily Available for Dental X-Ray Units, and What Components Typically Need Replacement?
Yes, reputable manufacturers and authorized distributors maintain comprehensive spare parts inventories for all major components, including X-ray tubes, sensors, control panels, collimators, and positioning arms. In 2026, modular design trends have improved part interchangeability, reducing downtime. Common wear items include X-ray tube assemblies (lifespan: 3–7 years, depending on usage), sensors (especially in intraoral units), and mechanical joints in C-arms. We recommend purchasing extended service contracts or stocking critical spares—particularly for high-volume clinics. Distributors should confirm parts availability and lead times before finalizing bulk orders.
3. What Does the Installation Process for a Dental X-Ray Machine Involve, and Is Professional Setup Required?
Professional installation by certified biomedical or dental equipment engineers is mandatory for all dental X-ray systems in 2026 due to regulatory and safety requirements. The process includes site assessment (space, power, wall anchoring), radiation shielding verification (if applicable), electrical connection, mechanical assembly (for floor-mounted or panoramic units), software calibration, and integration with existing imaging software (e.g., DICOM compatibility). Mobile and wall-mounted intraoral units require less setup but still need precise alignment and safety testing. Most manufacturers offer turnkey installation as part of the purchase agreement, especially for CBCT and panoramic systems.
4. What Warranty Coverage Is Standard for Dental X-Ray Machines in 2026, and What Does It Include?
As of 2026, most premium dental X-ray manufacturers offer a standard 2-year comprehensive warranty covering parts, labor, and technical support. Extended warranties (up to 5 years) are available for an additional cost and are highly recommended for high-throughput clinics. The warranty typically includes:
- Defects in materials and workmanship
- Failure of X-ray generator, sensor, and control system
- On-site service calls within 48 hours (region-dependent)
- Software updates and cybersecurity patches
Exclusions usually include damage from power surges, improper handling, or unauthorized modifications. Distributors should verify warranty transferability for resale and confirm global service network access for international clients.
5. How Do Voltage Fluctuations Impact Warranty Claims, and Can I Protect My Investment?
Repeated voltage fluctuations or power surges can void the warranty if they cause damage to the X-ray generator or digital components, as such events are classified as external environmental factors. In 2026, many manufacturers require proof of proper power conditioning (e.g., surge protectors or uninterruptible power supplies) during warranty claim assessments. To protect your investment, clinics should install medical-grade voltage stabilizers and ensure proper grounding. Distributors are advised to include power protection solutions as part of the equipment package, especially in regions with unstable grids, to reduce service calls and enhance customer satisfaction.
Specifications subject to change based on regulatory updates and technological advancements.
Need a Quote for Dental X-Ray Machine Prices?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


