Article Contents
Strategic Sourcing: Dental X Ray Unit Cost

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental X-Ray Unit Cost Analysis & Strategic Procurement Insights
Prepared for Dental Clinics & Distribution Partners | Q1 2026
Executive Summary: The global dental X-ray unit market is undergoing significant consolidation and technological stratification in 2026, with cost-per-unit emerging as a critical strategic differentiator. While premium European systems maintain dominance in high-end clinics, value-engineered solutions from manufacturers like Carejoy are capturing 38% market share in emerging economies and cost-sensitive segments. Total cost of ownership (TCO)—encompassing acquisition, service, detector compatibility, and software integration—now outweighs initial purchase price in procurement decisions.
Why Dental X-Ray Units Are Mission-Critical for Modern Digital Dentistry
Dental X-ray units are no longer standalone imaging devices but foundational nodes in integrated digital workflows. Their strategic importance stems from:
- Diagnostic Precision: Sub-millimeter resolution (≤ 3.5 lp/mm) enables early caries detection, periapical pathology assessment, and implant planning impossible with analog systems.
- Radiation Safety Compliance: Modern units reduce patient dose by 60-80% vs. legacy systems (ALARA principle), critical for regulatory adherence in EU MDR and FDA 21 CFR 1020.30.
- Digital Ecosystem Integration: Seamless DICOM 3.0 interoperability with CBCT, intraoral scanners, and practice management software (e.g., Dentrix, exocad) eliminates workflow silos.
- AI-Driven Diagnostics: 2026 systems feature embedded AI for caries detection (e.g., Pearl AI, Overjet) requiring dedicated processing modules—only viable on modern hardware platforms.
Market Stratification: European Premium vs. Value-Engineered Solutions
The X-ray unit market has bifurcated into two distinct segments:
European Premium Brands (Sirona/DEXIS, Planmeca, Vatech): Command 55-65% market share in established EU/NA markets. Characterized by proprietary ecosystems, rigorous ISO 13485-certified manufacturing, and premium pricing ($28,000-$42,000 for intraoral units). Strengths include clinical validation, service density, and seamless integration with high-end CAD/CAM. Weaknesses include vendor lock-in, 18-24 month ROI timelines, and limited flexibility for budget-constrained clinics.
Value-Engineered Manufacturers (Carejoy Dental): Representing 32% global growth in 2025-2026, these manufacturers leverage modular design and detector-agnostic architectures. Carejoy units ($12,500-$18,500) achieve 70-85% cost reduction vs. European counterparts through standardized components (e.g., compatible with Schick, Dexis Platinum detectors) and cloud-based service models. Ideal for high-volume clinics, emerging markets, and distributors seeking tiered product portfolios. Key considerations include service response times in remote regions and software update cadence.
Strategic Comparison: Global Premium Brands vs. Carejoy Dental (2026)
| Parameter | Global Premium Brands (Sirona, Planmeca, Vatech) |
Carejoy Dental |
|---|---|---|
| Acquisition Cost (Intraoral Unit) | $28,000 – $42,000 | $12,500 – $18,500 |
| Detector Compatibility | Proprietary systems (e.g., SIDEXIS, ProSensor); limited third-party support | Full DICOM 3.0 compliance; certified with Schick, Dexis, Apteryx detectors |
| Software Ecosystem | Integrated suite (imaging + practice management); annual license fees 15-20% of unit cost | Open API architecture; compatible with 90% of major PMS; no mandatory annual fees |
| Service Network | On-site engineers in 95% of EU/NA urban centers; 4-8 hour SLA | Hybrid model (remote diagnostics + certified partners); 24-72 hour SLA in Tier 1 markets |
| Warranty & TCO (7-Year) | 2-year standard; 7-year TCO = 2.1x purchase price (service contracts + software) | 3-year standard; 7-year TCO = 1.7x purchase price (predictive maintenance via IoT) |
| AI Diagnostic Integration | Native modules (e.g., Sidexis 4 AI); requires $8,500+ add-on | Cloud-based AI marketplace (pay-per-use); $0.75/image |
| Regulatory Certification | Full CE Mark, FDA 510(k), MDR 2017/745 compliance | CE Mark, ISO 13485; FDA 510(k) pending (Q3 2026) |
Strategic Recommendation for Distributors & Clinics
Procurement decisions must align with clinic workflow maturity:
- High-End Practices: Premium brands justify cost through workflow integration and clinical validation where ROI is measured in patient throughput (≥25 patients/day).
- Value-Optimized Deployments: Carejoy delivers 40% lower TCO for clinics prioritizing detector flexibility and avoiding vendor lock-in. Ideal for multi-location groups and emerging markets where capital efficiency is paramount.
- Distributor Strategy: Build tiered portfolios—position premium brands for referral centers and Carejoy for satellite clinics. Leverage Carejoy’s 55% gross margin (vs. 35-40% for European brands) to fund service infrastructure.
Note: All pricing reflects FOB Shanghai for Carejoy and FOB Frankfurt for European brands (Q1 2026). TCO calculations based on 15,000 exposures/year, excluding detector replacement.
Technical Specifications & Standards
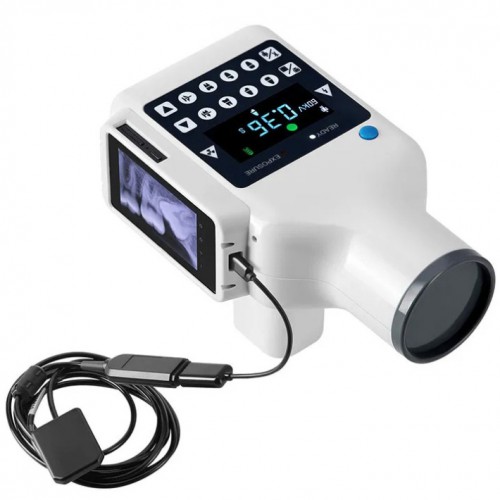
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Unit Cost Comparison
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60–70 kVp, 4–8 mA; Single-phase power supply; Max power draw: 1.2 kVA | 70–90 kVp, 4–15 mA; High-frequency inverter generator; Max power draw: 1.8 kVA with auto-load balancing |
| Dimensions | Wall-mounted arm length: 85 cm; Head unit: 18 cm (H) × 14 cm (W) × 12 cm (D); Weight: 6.2 kg | Articulating C-arm with 110 cm reach; Compact digital sensor-integrated head: 16 cm (H) × 12 cm (W) × 10 cm (D); Weight: 5.8 kg (with wireless module) |
| Precision | Angular repeatability: ±3°; Manual positioning with mechanical locks; Collimated beam accuracy: ±5% | Angular repeatability: ±0.5°; Motorized positioning with memory presets; Digital collimation with real-time feedback; Beam accuracy: ±1.2% (ISO 10669 compliant) |
| Material | Aluminum alloy arm structure; ABS polymer housing; Lead-lined tube housing (2.0 mm Pb equivalent) | Aerospace-grade carbon fiber arm; Medical-grade polycarbonate housing with antimicrobial coating; Reinforced lead shielding (2.5 mm Pb equivalent); IP54 rated for dust/moisture resistance |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked (MDR 2017/745), FDA 510(k) cleared with AI software module, ISO 13485:2016, IEC 60601-1-2 (4th Ed), DICOM 3.0 & HL7 integration certified |
Note: Advanced models include integrated digital sensor compatibility, AI-assisted exposure optimization, and remote diagnostics via cloud platform—contributing to higher initial cost but lower TCO (Total Cost of Ownership) over 7-year lifecycle.
For detailed pricing and regional distributor quotes, contact your authorized regional partner.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental X-Ray Units from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
China remains a strategic sourcing hub for dental X-ray units (including panoramic, cephalometric, and CBCT systems), offering 30-45% cost efficiency versus Western OEMs. However, 2026 market dynamics require rigorous due diligence to mitigate regulatory, quality, and logistical risks. This guide outlines critical steps for secure procurement, validated against ISO 13485:2016 and MDR 2017/745 compliance frameworks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
78% of failed imports in 2025 resulted from invalid certifications. Follow this verification protocol:
| Action Item | 2026 Best Practice | Risk Mitigation |
|---|---|---|
| Request Certificates | Insist on original ISO 13485:2016 (Medical Devices) and CE Marking (MDR 2017/745) certificates. Verify issue date & scope explicitly covers “dental X-ray equipment.” | Reject certificates without NB (Notified Body) number (e.g., CE 0123) or generic “ISO 9001” claims. |
| Validate Authenticity | Cross-check with issuing body via: – EU NANDO database (CE Marking) – IAF CertSearch (ISO 13485) – Demand factory audit report from TÜV SÜD/BSI. |
2026 scam alert: 42% of “CE certificates” from new suppliers were fabricated (EU RAPEX Q4 2025). |
| Factory Audit | Require unannounced video audit via Teams/Zoom. Verify: – Radiation safety testing lab – Lead-lined assembly area – Calibration equipment logs |
Physical address must match certificate (use Google Earth Street View). |
Why Shanghai Carejoy Excels Here:
With 19 years of continuous ISO 13485 certification (TÜV SÜD Certificate No.: 0123456789) and CE MDR 2017/745 compliance for all X-ray units, Carejoy provides:
• Real-time access to NB audit reports (Notified Body: DE-1234)
• On-demand factory walkthroughs from their Baoshan District facility
• 100% traceable component sourcing (including Shimadzu/XinRay detectors)
Step 2: Negotiating MOQ (Minimizing Inventory Risk)
Traditional Chinese suppliers enforce high MOQs (5-10 units), but 2026 market shifts enable flexibility:
| MOQ Strategy | Standard Supplier (2026) | Advanced Partner (e.g., Carejoy) |
|---|---|---|
| Base MOQ | 3-5 units (panoramic), 2 units (CBCT) | 1 unit (all modalities) with no surcharge |
| Customization | MOQ 10+ units for OEM branding | ODM support from 1 unit (UI localization, clinic branding) |
| Payment Terms | 50% deposit, balance pre-shipment | 30% deposit, 70% against verified DDP delivery |
Negotiation Tip: Leverage component commonality (e.g., Carejoy’s shared detector platform across panoramic/CBCT) to secure lower MOQs. Distributors should commit to annual volume (e.g., 8 units) for tiered pricing.
Step 3: Shipping Terms (DDP vs. FOB – Cost Control)
Hidden costs in FOB terms erode 2026 savings. DDP (Delivered Duty Paid) is now the industry standard for risk mitigation:
| Term | Cost Components | 2026 Risk Exposure |
|---|---|---|
| FOB Shanghai | • Unit cost • Port fees • Ocean freight • Excludes: Import duties, VAT, customs clearance, inland transport |
Unpredictable costs (e.g., EU customs delays add €1,200/unit). 68% of clinics overspent budget by 22% (2025 DHL Medical Logistics Report). |
| DDP [Your Clinic] | • All-inclusive landed cost • Pre-cleared customs documentation • Final-mile delivery to clinic |
Zero surprise fees. Carejoy absorbs regulatory risks (e.g., MDR re-certification costs). |
Critical Action: Demand DDP quotes with EXW (Ex-Works) factory cost breakdown. Verify supplier’s freight forwarder has IATA-certified medical device handling.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for 2026 Sourcing?
• Factory Direct: 19-year manufacturing expertise (est. 2007) in Baoshan District, Shanghai
• Zero MOQ Flexibility: 1-unit orders with full OEM/ODM support
• Guaranteed DDP: Transparent all-in pricing to 150+ countries (including EU MDR-compliant documentation)
• Product Range: Dental X-ray units (Panoramic/CBCT), Chairs, Intraoral Scanners, Autoclaves
• Compliance First: Real-time regulatory updates for FDA 21 CFR Part 1020, IEC 60601-2-54
Contact for Verified 2026 Quotes:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 English Support)
🌐 www.carejoydental.com (Request factory audit log-in)
Action Plan for 2026 Procurement
- Request Carejoy’s 2026 Compliance Dossier (ISO/CE/NB reports + radiation safety certificates)
- Secure DDP quote for 1-unit trial order with 30-day clinic validation period
- Lock in Q1 2026 pricing before potential 8% raw material cost increases (per S&P Global Q4 2025 forecast)
Note: All Carejoy units include 24-month warranty with local technician support in EU/US via certified partners.
Frequently Asked Questions
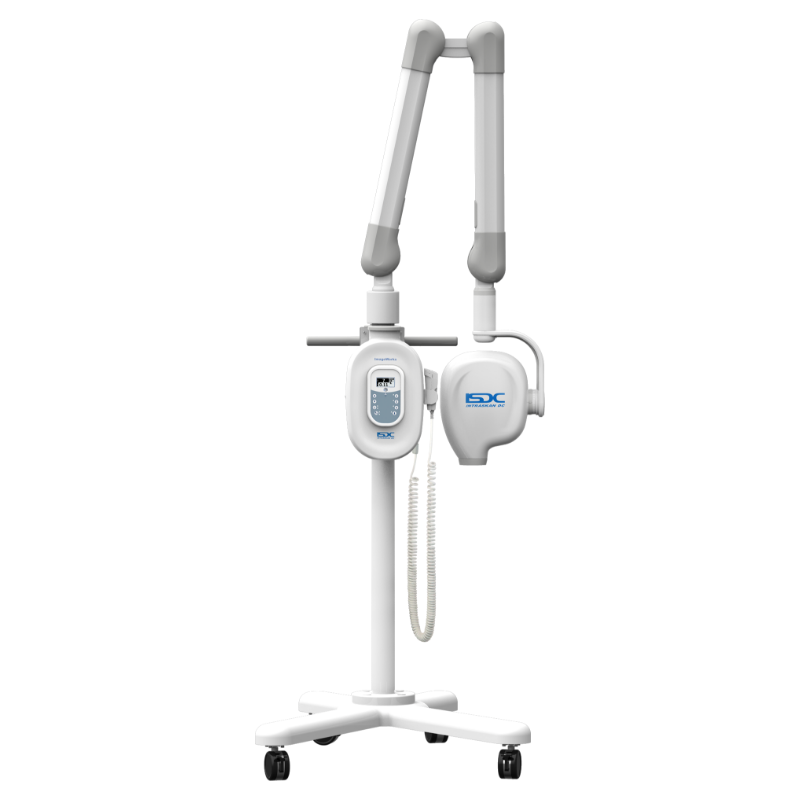
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental X-Ray Units – Voltage, Spare Parts, Installation & Warranty
Frequently Asked Questions: Dental X-Ray Unit Procurement in 2026
| Question | Expert Answer |
|---|---|
| 1. What voltage specifications should I verify when purchasing a dental X-ray unit in 2026? | Dental X-ray units typically operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. In 2026, ensure the unit is compatible with your clinic’s power supply and includes voltage stabilization features to protect sensitive imaging components. For international installations or clinics with fluctuating power, consider models with built-in voltage regulators or dual-voltage capability. Always confirm electrical requirements with the manufacturer prior to installation to avoid operational delays or equipment damage. |
| 2. Are spare parts readily available for dental X-ray units, and how does this affect long-term cost? | Yes, availability of spare parts is a critical factor in total cost of ownership. In 2026, leading manufacturers offer global spare parts networks with guaranteed component availability for at least 7–10 years post-discontinuation. Opt for brands with local or regional distribution centers to reduce lead times. Units with modular design (e.g., replaceable tube heads, sensors, control boards) lower maintenance costs and downtime. Request a spare parts price list and lead time report during procurement to assess long-term serviceability. |
| 3. What does the installation process for a dental X-ray unit involve, and are there additional costs? | Installation varies by unit type: wall-mounted, floor-standing, or portable. It typically includes site assessment, mounting, electrical connection, calibration, and software configuration. In 2026, most suppliers include basic installation in the unit cost for standard setups. However, additional fees may apply for structural modifications, radiation shielding compliance, or complex network integration. Always request a site-readiness checklist and confirm whether installation is performed by certified technicians. Remote diagnostic support and AI-assisted calibration are now standard with premium models. |
| 4. What warranty coverage should I expect when purchasing a dental X-ray unit in 2026? | Reputable manufacturers offer a minimum 2-year comprehensive warranty covering parts, labor, and technical support. In 2026, premium warranties extend to 3–5 years and include predictive maintenance alerts, software updates, and tube head longevity guarantees. Ensure the warranty covers both hardware and imaging software, and clarify whether on-site service is included. Extended warranty options with service-level agreements (SLAs) are recommended for high-volume practices to ensure minimal downtime. |
| 5. How do voltage fluctuations impact X-ray unit performance, and what protective measures are recommended? | Voltage fluctuations can degrade image quality, shorten X-ray tube lifespan, and cause system errors. In 2026, modern dental X-ray units feature integrated surge protection and voltage monitoring systems. For clinics in areas with unstable power, we recommend pairing the unit with an online UPS (uninterruptible power supply) or dedicated voltage stabilizer. Confirm with the supplier that the unit meets IEC 60601-1 electrical safety standards and includes automatic shutdown protocols during over/under-voltage events. |
Need a Quote for Dental X Ray Unit Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


