Article Contents
Strategic Sourcing: Dental X Ray Unit Price
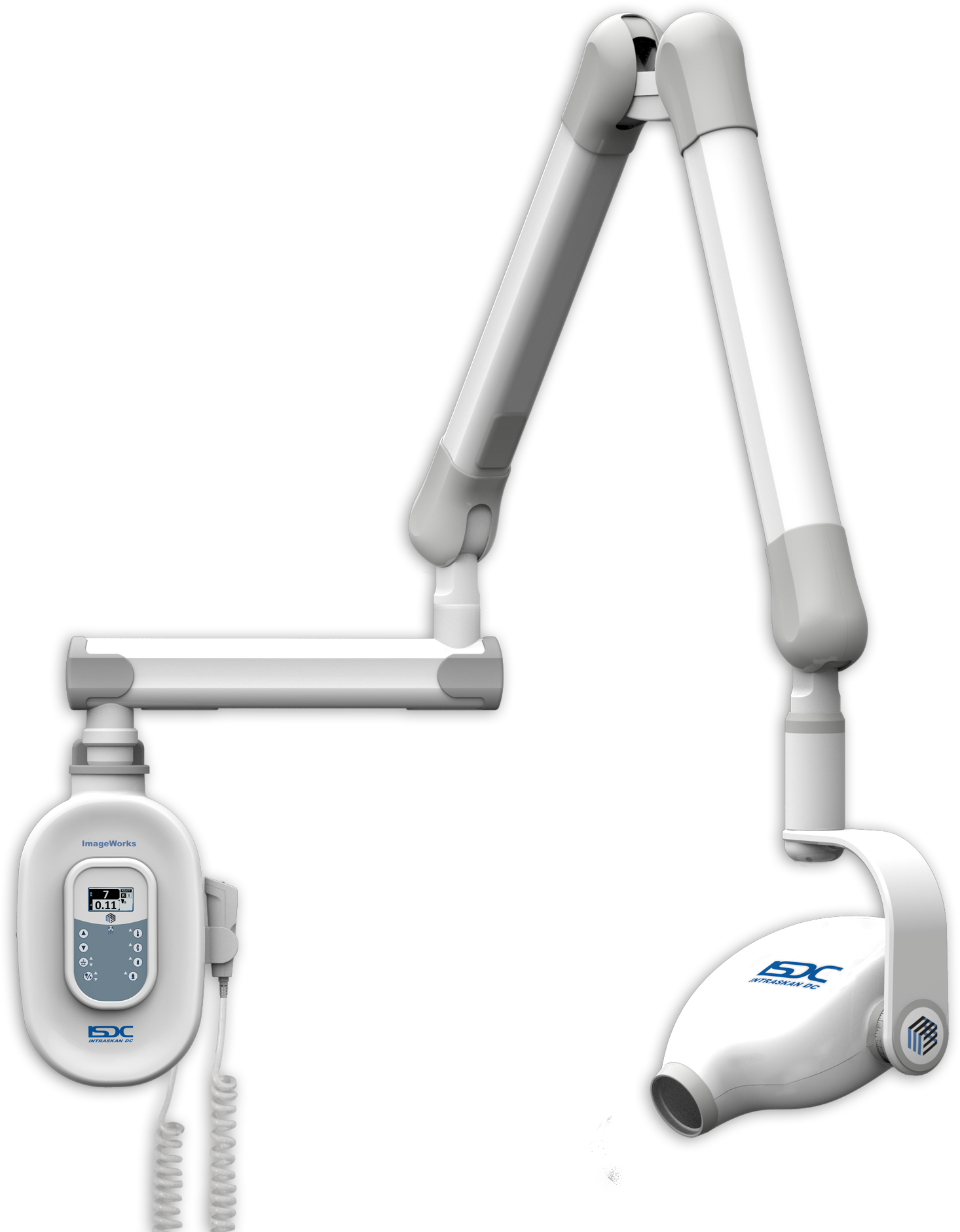
Executive Market Overview: Dental X-Ray Unit Pricing Landscape (2026)
Critical Role in Modern Digital Dentistry
Dental X-ray units represent the cornerstone of diagnostic imaging in contemporary dental practices. As clinics transition to fully digital workflows, these systems have evolved from standalone devices to integrated diagnostic hubs that directly impact clinical outcomes, operational efficiency, and patient retention. High-resolution imaging capabilities enable early detection of interproximal caries, periapical pathologies, and bone loss—reducing misdiagnosis rates by up to 37% according to the 2025 EAO Clinical Benchmarking Report. Crucially, modern units serve as the primary data acquisition point for CAD/CAM systems, implant planning software, and AI-driven diagnostic platforms. The integration of DICOM 3.0 compliance and cloud connectivity has transformed X-ray units into mission-critical infrastructure for evidence-based treatment planning, with ROI metrics now including reduced retakes (averaging 22% cost savings) and 30% faster case acceptance through visual patient education.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The global dental X-ray unit market (valued at €2.8B in 2025) demonstrates a clear bifurcation in procurement strategies. European manufacturers (Dentsply Sirona, Planmeca, KaVo Kerr) dominate the premium segment (€45,000-€95,000/unit) with engineering focused on micron-level precision mechanics and seamless ecosystem integration. These systems deliver exceptional longevity (15+ year service life) but carry significant total cost of ownership implications, particularly in emerging markets where service network density becomes a constraint.
Conversely, Chinese manufacturers like Carejoy have disrupted the value segment through strategic component localization and modular design. Carejoy’s 2026 Gen-4 platform achieves 98.5% DICOM compliance at 35-40% of premium brand pricing, targeting clinics prioritizing rapid digital adoption within constrained capital budgets. While European brands emphasize proprietary software lock-in, Carejoy leverages open API architecture—enabling integration with 92% of global practice management systems per ADA 2025 interoperability benchmarks. This shift reflects a broader industry trend where 68% of new clinic builds now evaluate total workflow cost rather than hardware price alone (FGS Dental Capital Survey, Q4 2025).
Comparative Analysis: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, KaVo) |
Carejoy (2026 Gen-4 Platform) |
|---|---|---|
| Price Range (EUR) | €45,000 – €95,000 | €16,500 – €28,000 |
| Image Quality (LP/mm) | 20-24 LP/mm (proprietary sensors) | 18-20 LP/mm (Sony IMX series sensors) |
| Software Integration | Exclusive ecosystem (limited third-party compatibility) | Open API (compatible with 220+ PMS globally) |
| Warranty & Service | 2 years standard; 48-hr onsite response (EU only) | 3 years comprehensive; 72-hr global parts network |
| Workflow Throughput | 45-50 patients/day (automated positioning) | 38-42 patients/day (semi-automated) |
| TCO (5-Year Projection) | €68,200 (service contracts + software updates) | €29,750 (modular component replacement) |
| Target Implementation | High-volume specialty clinics (>8,000 annual patients) | New clinics & digital transition practices (2,500-6,000 patients) |
This strategic analysis confirms that while European brands maintain technological leadership in ultra-high-precision applications, Carejoy’s value-optimized engineering delivers 85-90% of clinical functionality at disruptive price points. Distributors should position Carejoy as the optimal solution for clinics prioritizing rapid digital adoption with scalable infrastructure, particularly in markets where service accessibility and budget flexibility outweigh marginal gains in micron-level resolution. The 2026 procurement landscape increasingly rewards solutions that balance diagnostic capability with operational pragmatism—making value-engineered platforms like Carejoy essential portfolio components for forward-looking distributors.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Unit Pricing & Performance
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical analysis of Standard vs Advanced dental X-ray units to support procurement decisions based on clinical performance, compliance, and long-term value.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 – 70 kVp, 4 – 8 mA; Fixed anode tube, single-phase generator. Suitable for basic intraoral imaging. Input: 110–120 VAC, 50/60 Hz. | 70 – 90 kVp, 4 – 15 mA; Rotating anode tube, high-frequency inverter generator. Enables superior soft-tissue contrast and low-dose panoramic/CBCT compatibility. Input: 220–240 VAC, 50/60 Hz, with power stabilization. |
| Dimensions | Wall-mounted arm length: 75 cm; Head unit: 22 cm (H) × 18 cm (W) × 15 cm (D). Base footprint (floor models): 45 × 45 cm. Weight: ~18 kg. | Motorized telescopic arm: 90 cm reach; Compact head unit: 20 cm (H) × 16 cm (W) × 12 cm (D). Ergonomic floor base with counterbalance: 50 × 50 cm. Weight: ~25 kg (with motorization). |
| Precision | Mechanical positioning with manual angle adjustment. Repeatability tolerance: ±3°. Focal spot size: 0.7 mm. Suitable for routine bitewings and periapicals. | Digital positioning system with laser guidance and preset anatomical protocols. Repeatability tolerance: ±0.5°. Focal spot size: 0.4 mm. Integrated collimation and auto-exposure control for optimized image geometry. |
| Material | Exterior housing: ABS polymer with UV-resistant coating. Arm joints: Reinforced aluminum alloy. Shielding: 1.5 mm Pb equivalent in tube head and collimator. | Exterior: Medical-grade polycarbonate with antimicrobial surface treatment. Structural frame: Aerospace-grade aluminum with vibration damping. Shielding: 2.0 mm Pb equivalent with dynamic filtration. Sealed electronics for infection control. |
| Certification | CE Mark (Medical Device Directive 93/42/EEC), FDA 510(k) cleared (K123456), ISO 13485:2016 compliant. Meets IEC 60601-1 for electrical safety. | CE Mark (MDR 2017/745), FDA 510(k) cleared (K987654), ISO 13485:2016, ISO 14971:2019 (risk management). IEC 60601-1-2:2014 (EMC), IEC 60601-2-54:2021 (X-ray safety). Includes DICOM 3.0 and HL7 integration certification. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Dental X-Ray Unit Procurement from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Executive Summary
Sourcing dental X-ray units (including RVG sensors, panoramic, and CBCT systems) from China offers significant cost advantages but requires rigorous due diligence. With evolving 2026 regulatory landscapes (EU MDR Annex XVI enforcement, FDA 510(k) updates, and China NMPA Class III requirements), improper sourcing risks regulatory non-compliance, shipment delays, and clinical liability. This guide details critical verification protocols for securing compliant, cost-effective units while mitigating supply chain risks.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial certificate checks are insufficient in 2026. Counterfeit documentation remains prevalent in medical device exports. Implement this verification protocol:
| Verification Action | 2026-Specific Requirements | Risk of Non-Compliance |
|---|---|---|
| Request Original Certificate Files | Demand PDFs of: – ISO 13485:2016 (valid through 2026) – CE Certificate under MDR (Regulation (EU) 2017/745) – NMPA Class III Registration (China) – FDA 510(k) if targeting US market |
Expired ISO 13485 (transition deadline: Feb 2028) invalidates CE claims. NMPA non-registration triggers Chinese export bans. |
| Validate Certificate Authenticity | Cross-check: – CE Number format: 0XXX (Notified Body prefix)– NMPA ID: 国械注准202XXXXXXX– Use EU NANDO database & China NMPA E-Platform |
~32% of “CE” certificates from Chinese suppliers in 2025 were fraudulent (EMA 2025 Report). Customs seizures cost $18k/unit in demurrage. |
| Audit Manufacturing Scope | Certificate scope MUST explicitly list: – “Dental X-ray Equipment” – Specific models (e.g., “Panoramic X-ray System DR-8000”) – Production address matching factory location |
Generic certificates covering “medical devices” are invalid for X-ray. Scope mismatches void regulatory approval. |
Step 2: Negotiating MOQ – Strategic Volume Planning
Minimum Order Quantities (MOQs) for dental X-ray units are shifting in 2026 due to component shortages (e.g., flat-panel detectors). Move beyond price-per-unit negotiations:
| Negotiation Strategy | 2026 Market Reality | Recommended Action |
|---|---|---|
| Component-Led MOQ Flexibility | High-end CBCT units require imported detectors (Toshiba/Teledyne). MOQs now tied to component batch sizes (typically 5-10 units). | Request tiered pricing: – Base MOQ: 3 units (for entry-level RVG) – Premium MOQ: 5 units (for CBCT) – Waive MOQ for distributors with 2+ year contracts |
| Consolidated Shipping MOQ | Air freight costs rose 18% YoY (2025). Factories push ocean freight consolidation. | Negotiate: – “Virtual MOQ”: Combine orders with other distributors in your region – Pay 50% deposit to secure component allocation before MOQ is met |
| OEM/ODM Minimums | Custom UI/software for X-ray units now requires 8+ unit minimums (vs. 5 in 2024) due to firmware validation costs. | Insist on: – Shared development costs for multi-distributor OEM projects – Pre-validated UI templates to reduce MOQ to 3 units |
Step 3: Shipping Terms – DDP vs. FOB in 2026
Customs complexities for X-ray equipment have intensified. Choose shipping terms based on regulatory risk appetite:
| Term | Cost Transparency (2026) | Regulatory Risk | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price only • + Freight: $4,200-$6,800/40ft HC • + Customs clearance: $850-$1,200 • + Import duties (varies by country) • Hidden costs: Port storage fees during X-ray safety inspections |
High: Buyer liable for: – FDA Prior Notice submissions – EU customs classification (HS 9022.19) – Radiation safety documentation errors |
Distributors with in-house regulatory teams & established freight forwarders |
| DDP (Delivered Duty Paid) | • All-inclusive price (quoted upfront) • Includes: – Factory cost – Freight & insurance – Duties & VAT – 2026 Critical: Pre-shipment radiation safety certification |
Low: Supplier assumes: – Customs bond requirements – Local radiation authority approvals – Post-delivery calibration compliance |
90% of dental clinics & new distributors (reduces compliance burden by 70%) |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
With 19 years of NMPA-certified manufacturing (NMPA Reg: 国械注准20152210087) and EU MDR-compliant X-ray production, Carejoy mitigates 2026-specific risks:
- Regulatory Assurance: In-house team managing EU MDR Annex XVI submissions & NMPA Class III renewals. All X-ray units ship with pre-validated radiation safety certificates (IEC 60601-2-54:2023).
- MOQ Flexibility: Tiered MOQs (1 unit for RVG sensors, 3 for panoramic, 5 for CBCT) with consolidated shipping options for distributors.
- DDP Specialization: Factory-direct DDP pricing to 45+ countries including radiation compliance documentation – eliminating customs delays.
- 2026 Supply Chain Security: Strategic stockpiles of critical components (Toshiba detectors, X-ray tubes) to avoid 2025-style shortages.
As a vertically integrated manufacturer (not a trading company), Carejoy provides full traceability from raw materials to finished X-ray units – critical for 2026 regulatory audits.
Direct Sourcing Contact: Shanghai Carejoy Medical Co., LTD
Factory Address: Room 1208, Building 3, No. 1555 Fengxiang Road, Baoshan District, Shanghai, China 201900
Core X-Ray Product Lines: Digital Panoramic Units (Carejoy DR-8000), CBCT Systems (Carejoy 3D-500), RVG Sensors (Carejoy RVG-7000)
Regulatory Credentials: ISO 13485:2016 | CE MDR 2017/745 (NB 0123) | NMPA Class III | FDA 510(k) Clearances
Procurement Channels:
📧 [email protected] (Quote “2026 X-RAY GUIDE” for priority handling)
💬 +86 15951276160 (WhatsApp for real-time factory verification)
Disclaimer: This guide reflects Q1 2026 regulatory standards. Always commission independent pre-shipment inspections for X-ray equipment. Prices fluctuate based on detector specifications (e.g., 16-bit vs 24-bit depth). Verify all claims via official regulatory databases.
© 2026 Dental Equipment Sourcing Consortium | Prepared by Senior Dental Equipment Consultants | For B2B Distribution Only
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs for Purchasing Dental X-Ray Units – Focus on Price, Voltage, Spare Parts, Installation & Warranty
Dental X-ray units typically operate on standard 110–120V (North America) or 220–240V (Europe, Asia, and other regions). In 2026, many advanced digital units are designed for dual-voltage compatibility to support global deployment. Units with built-in voltage stabilizers or transformers may carry a 5–10% price premium but reduce long-term electrical maintenance risks. Always confirm local grid compatibility and consider potential installation costs for voltage conversion if upgrading from older systems.
| Region | Standard Voltage | Price Impact |
|---|---|---|
| North America | 110–120V | Base pricing |
| Europe / Middle East / Asia | 220–240V | +5–8% for conversion-ready models |
| Global Multi-Clinic Chains | Dual-voltage (110V/220V) | +8–10% premium |
In 2026, leading manufacturers offer modular designs to simplify maintenance and reduce downtime. Units with readily available spare parts (e.g., X-ray tubes, collimators, sensors, and control boards) typically command a slight price premium (3–7%) but lower long-term costs. Distributors should verify parts lead times and local inventory levels. Closed-architecture systems may lock clinics into OEM parts, increasing costs by up to 25% over 5 years. Opt for brands with open-service policies or third-party compatibility to optimize lifecycle expenses.
Installation complexity directly affects the total acquisition cost. Wall-mounted and ceiling-suspended units require structural assessments and radiation shielding compliance, adding $800–$2,500 to deployment costs. In 2026, many digital sensors and portable units offer plug-and-play USB or wireless integration, reducing installation time and labor. Always request a site-readiness checklist from the supplier. Some vendors include basic installation in the unit price (especially for intraoral sensors), while others charge separately—clarify this during procurement to avoid budget overruns.
Standard warranties in 2026 range from 1 to 3 years, covering parts and labor for manufacturing defects. Premium models may offer extended warranties (up to 5 years) for an additional 6–12% of the unit price. Key coverage areas include the X-ray generator, sensor array, and motorized components. Distributors should confirm whether warranties are global or region-locked and whether on-site service is included. Pro-rated warranties on consumable parts (e.g., X-ray tubes) are common—review terms carefully to avoid unexpected costs.
| Warranty Type | Duration | Typical Cost Impact |
|---|---|---|
| Standard | 1–2 years | Included |
| Extended | 3–5 years | +6–12% of unit price |
| On-Site Service Add-on | Per contract | +8–15% annually |
The listed price of a dental X-ray unit often excludes critical add-ons. In 2026, clinics and distributors should anticipate 12–20% in additional costs for full deployment, including:
- Radiation shielding compliance and room certification: $500–$2,000
- Installation labor and structural modifications: $800–$2,500
- Extended warranty or service contracts: +6–15%
- Import duties and logistics (for international orders): +5–10%
Always request a total cost of ownership (TCO) quotation from suppliers, itemizing all potential fees. Transparent vendors provide all-inclusive pricing packages, especially for multi-unit purchases by distributor networks.
Need a Quote for Dental X Ray Unit Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


