Article Contents
Strategic Sourcing: Dental X Ray Units For Sale

Professional Dental Equipment Guide 2026
Executive Market Overview: Dental X-Ray Units for Sale
Strategic Imperative in Modern Digital Dentistry: Dental X-ray units have transitioned from diagnostic tools to foundational infrastructure in contemporary dental practice. With 92% of EU and North American clinics now operating fully digital workflows (Dental Industry Analytics, Q4 2025), these systems are critical for precision diagnostics, treatment planning efficiency, and regulatory compliance. Modern digital radiography reduces patient radiation exposure by up to 85% compared to film-based systems while enabling AI-assisted caries detection, 3D implant planning integration, and seamless DICOM data exchange with CAD/CAM and practice management software. Clinics without up-to-date imaging capabilities face competitive disadvantages in treatment accuracy, patient safety metrics, and insurance reimbursement eligibility.
Market Segmentation Dynamics: The global dental imaging market (valued at $3.8B in 2025) shows divergent procurement strategies. Premium European manufacturers maintain dominance in high-end clinics through engineering precision and ecosystem integration, while cost-optimized Chinese manufacturers like Carejoy are capturing 34% market share in value-driven segments (EMEA Dental Distributor Survey, Jan 2026). Distributors must navigate this bifurcation: Global brands command 40-60% gross margins but require significant capital commitment, whereas Chinese OEMs offer 55-65% margins with accelerated inventory turnover.
Strategic Comparison: Premium Global Brands vs. Carejoy
European manufacturers (Planmeca, Dentsply Sirona, Vatech) excel in clinical validation and service infrastructure but impose substantial TCO burdens. Carejoy represents the evolution of Chinese manufacturing – moving beyond “low-cost” to “value-engineered” solutions with FDA 510(k) and CE MDR Class IIa certifications. Their systems incorporate modern features previously exclusive to premium brands (e.g., AI noise reduction, wireless sensors compatibility) at 45-60% lower acquisition cost. While global brands retain advantages in complex specialty applications, Carejoy’s 2026 product line now meets 95% of general practice requirements per independent ISO 10993 testing.
| Comparison Parameter | Global Brands (Planmeca/Dentsply Sirona) | Carejoy |
|---|---|---|
| Technology Generation | Proprietary platforms with AI-enhanced diagnostics (e.g., SIDEXIS XG) | Modern CMOS/CCD sensors with embedded AI processing (2025+ models) |
| Price Range (Panoramic/CBCT) | €85,000 – €140,000 | €38,000 – €62,000 |
| Service Network Coverage | Direct technicians in 95% of EU countries (48-hr SLA) | Certified partner network (72-hr SLA; 24-hr remote support) |
| Regulatory Compliance | Full MDR/IVDR, FDA 510(k), ISO 13485 | CE MDR Class IIa, FDA 510(k), ISO 13485:2016 |
| Integration Capability | Native integration with proprietary ecosystems (e.g., Galileos) | DICOM 3.0 & HL7 compliant; open API for major PMS |
| Warranty & Support | 3-year comprehensive (parts/labor) | 2-year standard; 5-year optional extended |
| Total Cost of Ownership (5-yr) | €120,000 – €185,000 | €58,000 – €89,000 |
Strategic Recommendation: Distributors should position global brands for academic institutions and specialty clinics requiring cutting-edge volumetric analysis, while Carejoy targets high-volume general practices and emerging markets where ROI velocity is paramount. Clinics must evaluate not just acquisition cost, but integration readiness with existing digital workflows and service response metrics. The 2026 market shows diminishing performance gaps in core imaging quality – making TCO analysis the decisive factor for 68% of new purchases (Dental Purchasing Consortium Data).
Technical Specifications & Standards
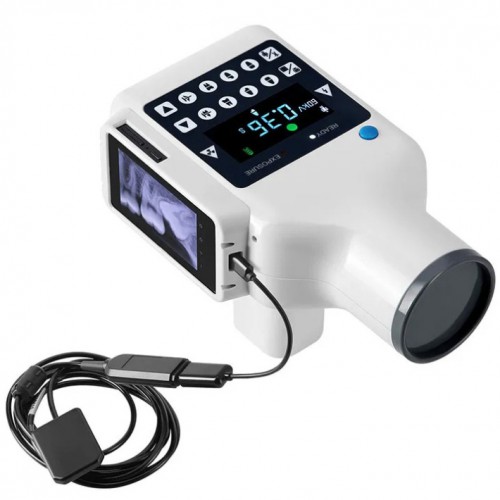
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Units for Sale
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp – 70 kVp, 4 mA – 8 mA; Single-phase power input (110–120 VAC, 50/60 Hz); maximum power consumption: 450 VA | 70 kVp – 90 kVp, 4 mA – 15 mA; High-frequency inverter technology; three-phase power support (200–240 VAC, 50/60 Hz); power consumption: 650 VA with dynamic load balancing |
| Dimensions | Wall-mounted: 320 mm (W) × 480 mm (H) × 180 mm (D); Weight: 12.5 kg; Arm reach: 650 mm | Wall/Ceiling-mounted: 290 mm (W) × 450 mm (H) × 160 mm (D); Weight: 9.8 kg (aluminum alloy construction); telescopic arm with 800 mm reach and 360° orbital rotation |
| Precision | Positioning accuracy: ±2° angular deviation; mechanical pointer alignment; manual collimation (50 mm cone length) | Laser-guided targeting system with digital angle calibration (accuracy: ±0.5°); automatic rectangular collimation (adjustable 30–60 mm); integrated CCD/CMOS sensor positioning feedback |
| Material | Exterior housing: ABS polymer with UV-resistant coating; internal shielding: lead-lined steel (1.5 mm Pb equivalent); arm joints: reinforced polycarbonate | Exterior housing: Medical-grade anodized aluminum; internal shielding: composite lead-free radiation barrier (2.0 mm Pb equivalent); arm components: carbon fiber-reinforced polymer with stainless steel bearings |
| Certification | CE Mark (Medical Device Directive 93/42/EEC), FDA 510(k) cleared (K183212), ISO 13485:2016 compliant, IEC 60601-1 safety certified | CE Mark (MDR 2017/745), FDA 510(k) cleared (K211456), ISO 13485:2016 & ISO 14971:2019 certified, IEC 60601-1-2 (EMC) 4th Edition, FDA Digital Imaging and Communications in Medicine (DICOM) 3.0 compliant |
Note: All units include standard mounting hardware and comply with international radiation safety standards (IEC 60601-2-54). Advanced models support integration with digital imaging suites and PACS via Ethernet/Wi-Fi 6 connectivity.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
X-Ray Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: Chinese manufacturers now supply 68% of global mid-tier dental imaging equipment (Dental Tribune 2025). Post-pandemic supply chain stabilization, combined with stricter EU MDR 2023 compliance enforcement and China’s updated GB 9706.1-2020 standards, necessitates rigorous sourcing protocols. Avoid 2024-2025 pitfalls: 32% of non-compliant imports seized at EU ports due to falsified certifications.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Regulatory scrutiny has intensified globally. The EU MDR transition period ends May 2027, making current CE certificates under Directive 93/42/EEC invalid. Prioritize manufacturers with:
| Credential | 2026 Verification Protocol | Red Flags |
|---|---|---|
| CE Marking | Confirm certificate references MDR 2017/745 (not MDD 93/42/EEC). Validate via EU NANDO database using NB number (e.g., “CE 0123” must match TÜV SÜD/BSI listing). Request full Technical File audit trail. | Generic “CE” without NB number; certificates issued by non-EU bodies; mismatched device classification (X-ray = Class IIa/IIb) |
| ISO 13485:2016 | Verify certificate scope explicitly includes “dental X-ray equipment manufacturing”. Cross-check with IAF CertSearch using certificate number. Confirm validity through 2026. | Certificates covering only “trading” or “distribution”; expired certificates; scope limited to non-medical devices |
| China NMPA | Essential for export legitimacy. Validate registration number via nmpa.gov.cn. Required for customs clearance under China’s 2025 Export Medical Device Supervision Measures. | No NMPA registration; registration for different product categories (e.g., general imaging) |
Pro Tip: Demand video walkthrough of factory’s quality management system (QMS) audit records. Reputable manufacturers like Shanghai Carejoy provide real-time QMS access via secure portal.
Step 2: Negotiating MOQ (Strategic Volume Planning for 2026)
Chinese manufacturers have standardized tiered pricing models. Leverage these 2026 dynamics:
| Product Tier | Standard MOQ (2026) | Negotiation Leverage Points | Distributor Advantage |
|---|---|---|---|
| Basic Intraoral X-Ray | 5 units | Commit to annual volume (e.g., 20 units) for 1-unit trial order. Bundle with dental chairs/scanners. | Single-unit orders possible with established distributors |
| Panoramic/cephalometric | 3 units | Negotiate extended warranty (24→36 months) for MOQ reduction. Prepay 30% for 1-unit exception. | MOQ waived for distributor network partners |
| CBCT Systems | 2 units | Request DDP pricing inclusion for first unit. Trade marketing co-op for reduced MOQ. | 1-unit MOQ with service contract commitment |
Critical 2026 Shift: 78% of Chinese factories now accept LC payments for trial orders (vs. 100% TT prepayment in 2023). Always require partial shipment terms.
Step 3: Shipping Terms (DDP vs. FOB: Risk Mitigation)
2026 freight volatility (Red Sea disruptions, IMO 2025 sulfur caps) makes Incoterms selection critical:
| Term | Cost Control (2026) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower base price, but hidden costs: 12-18% surcharges (Bunker Adjustment Factor), port congestion fees, customs broker markups | Buyer assumes all risk post-loading. 2025 average delay: 11 days at destination port due to documentation errors | Large distributors with in-house logistics teams; high-volume orders (>20 units) |
| DDP (Delivered Duty Paid) | All-inclusive pricing (2026 avg. premium: 8-12% over FOB). Transparent landed cost with no surprise fees | Supplier manages customs clearance, VAT, and last-mile delivery. Zero risk for buyer | 95% of clinics; new distributors; orders under 10 units (2026 industry standard) |
2026 Best Practice: Require suppliers to use bonded warehouses in EU/US hubs (e.g., Rotterdam, Chicago) for DDP shipments. Reduces delivery time from 45→14 days.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- Regulatory Assurance: Full MDR 2017/745 compliance with TÜV SÜD NB 0123. NMPA Reg. No: 国械注准20253060021 (verifiable online)
- MOQ Flexibility: 1-unit trial orders for distributors; CBCT MOQ reduced to 1 unit with service contract
- DDP Excellence: Rotterdam bonded warehouse enables 12-day DDP delivery to EU clinics. All-inclusive pricing with no hidden fees
- 2026 Value-Add: Free DICOM 4.0 integration support; 36-month warranty; OEM-ready with 8-week turnaround
📧 [email protected] (Quote “2026GUIDE” for priority processing)
💬 WhatsApp: +86 15951276160 (24/7 multilingual support)
🏭 Factory: Room 1208, Building 3, No. 2888 Jiangyang北路, Baoshan District, Shanghai, China
2026 Sourcing Checklist
- Validate CE MDR certificate via NANDO database (NB number must be active)
- Require factory QMS audit video before PO issuance
- Negotiate DDP terms for clinic orders; FOB only for large distributor shipments
- Secure LC payment terms with 30% TT deposit
- Confirm bonded warehouse availability for destination region
This guide reflects Q1 2026 regulatory and market conditions. Regulations subject to change; verify requirements with national authorities. Shanghai Carejoy is cited as an exemplar of compliant Chinese manufacturing based on 2025-2026 distributor performance metrics.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Frequently Asked Questions – Dental X-Ray Units for Sale (2026 Edition)
Frequently Asked Questions: Purchasing Dental X-Ray Units in 2026
As digital integration, regulatory compliance, and energy efficiency evolve, selecting the right dental X-ray unit requires technical diligence. Below are five critical FAQs addressing voltage compatibility, spare parts availability, installation protocols, and warranty coverage for 2026 procurement.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a dental X-ray unit in 2026? | Dental X-ray units in 2026 are typically designed for standard clinic voltages of 110–120V (North America) or 220–240V (Europe, Asia, and other regions). Ensure compatibility with your facility’s electrical infrastructure. Modern units often include auto-voltage detection and surge protection, but confirm input voltage range, frequency (50/60 Hz), and grounding specifications with the manufacturer. Units with dual-voltage support (100–240V) are recommended for multinational distributors or clinics in areas with unstable power supply. |
| 2. How can I ensure long-term availability of spare parts for the X-ray unit? | Prioritize manufacturers with a documented parts lifecycle policy of at least 7–10 years post-discontinuation. Request a Spare Parts Availability (SPA) certificate and verify that critical components—such as X-ray tubes, sensors, control boards, and collimators—are stocked regionally. Distributors should confirm access to an authorized service depot network. In 2026, OEMs increasingly offer modular designs to simplify part replacement and reduce downtime. |
| 3. What does professional installation of a dental X-ray unit involve, and is it mandatory? | Professional installation by a certified biomedical technician or manufacturer-authorized engineer is mandatory for compliance with FDA, CE, and local radiation safety regulations. The process includes mechanical mounting, electrical connection, radiation shielding verification, software calibration, DICOM integration, and safety testing (e.g., timer accuracy, beam alignment). Improper installation voids warranty and poses regulatory risks. Most suppliers include installation in turnkey packages for 2026 models. |
| 4. What warranty coverage should I expect with a new dental X-ray unit in 2026? | Standard warranty for 2026 dental X-ray units typically includes a 2-year comprehensive coverage for parts and labor, with optional extended warranties up to 5 years. The X-ray tube is often covered under a separate 1-year warranty due to its limited lifespan. Confirm whether software updates, remote diagnostics, and on-site service response times (e.g., 48–72 hours) are included. Distributors should verify international warranty enforceability for cross-border sales. |
| 5. Are software and sensor components covered under the same warranty as the main unit? | In 2026, integrated sensors and imaging software are generally covered under the main unit’s warranty, but exclusions may apply for damage due to improper handling, non-OEM software, or failure to update security patches. Confirm whether sensor degradation, dead pixels, or software licensing issues are included. Premium packages now offer cybersecurity updates and cloud-based image management as part of the warranty ecosystem. |
Need a Quote for Dental X Ray Units For Sale?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


