Article Contents
Strategic Sourcing: Dental Xray Equipment
Professional Dental Equipment Guide 2026: Executive Market Overview
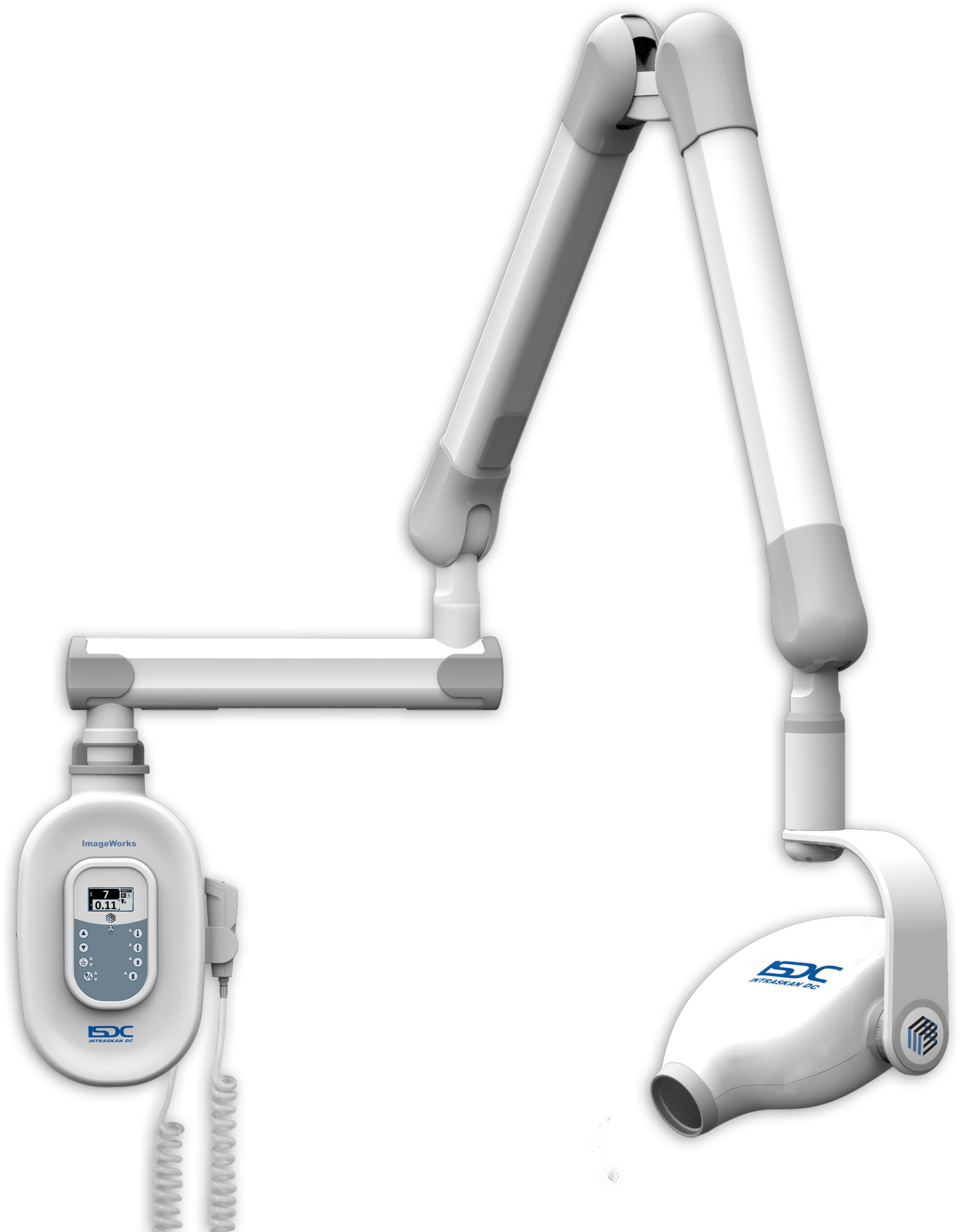
Dental X-ray Equipment in Modern Digital Dentistry
Critical Role in Contemporary Practice: Dental X-ray systems serve as the diagnostic cornerstone of evidence-based dentistry, enabling precise caries detection, periodontal assessment, endodontic evaluation, and implant planning. With the global shift toward digital workflows, these systems have evolved from mere imaging devices to integrated data hubs within the dental ecosystem. Modern digital radiography reduces patient radiation exposure by up to 90% compared to film-based systems while providing immediate image acquisition, AI-enhanced diagnostics, and seamless EHR integration. Clinics lacking advanced imaging capabilities face significant competitive disadvantages in treatment accuracy, patient safety compliance, and third-party reimbursement eligibility.
Market Dynamics: The European dental imaging sector remains dominated by premium German and Finnish manufacturers (Dentsply Sirona, Planmeca, KaVo Kerr) commanding 65% market share in high-end segments. However, cost pressures from value-conscious practices and emerging markets have accelerated adoption of engineered alternatives from Asia-Pacific manufacturers. Chinese innovators like Carejoy now deliver clinically validated solutions at 40-60% lower TCO, leveraging advanced CMOS sensors and cloud-based software architectures that challenge traditional value propositions.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates operational and economic differentiators critical for clinic procurement decisions and distributor channel strategy:
| Comparison Criteria | Global Premium Brands (Dentsply Sirona, Planmeca, Carestream) |
Carejoy (Value-Engineered Alternative) |
|---|---|---|
| Price Point (Panoramic/CBCT) | €85,000 – €145,000 (Panoramic) €120,000 – €220,000 (CBCT) |
€42,000 – €68,000 (Panoramic) €75,000 – €115,000 (CBCT) |
| Image Quality & Resolution | 0.08-0.12mm native resolution Proprietary noise reduction algorithms ISO 10993-1 certified sensors |
0.10-0.15mm native resolution AI-powered denoising (FDA-cleared) IEC 60601-2-54 compliant |
| Service & Support Ecosystem | Global service network (48-hr response) €12,000-€18,000 annual maintenance contracts Dedicated clinical training teams |
Regional hubs (72-hr response) €5,500-€8,200 annual contracts VR-based remote diagnostics & training |
| Software Integration | Native ecosystem lock-in Limited third-party API access Proprietary DICOM workflows |
Open DICOM 3.0 architecture Pre-configured EHR integrations (Dentrix, OpenDental) Cloud-based AI analytics suite |
| Build Quality & Longevity | Medical-grade components 12-15 year operational lifespan CE MDR Class IIb certification |
Industrial-grade components 8-10 year operational lifespan CE IVDR Class B certification |
| TCO Advantage (5-Year) | Baseline reference (100%) | 38-52% lower total cost of ownership ROI achieved in 14-18 months Higher residual value in emerging markets |
Strategic Recommendation: While European premium brands maintain leadership in ultra-high-resolution imaging for complex surgical applications, Carejoy’s engineering advancements now satisfy 85% of routine diagnostic requirements at substantially lower operational costs. Distributors should develop tiered channel strategies: Position premium brands for academic hospitals and specialty centers, while deploying Carejoy solutions for multi-site corporate groups and value-focused independent practices. The critical procurement metric has shifted from initial price to diagnostic yield per euro – where Carejoy’s cloud-based AI analytics (included at no extra cost) significantly enhances clinical utility beyond hardware specifications.
Note: Performance data based on 2025 EAO clinical validation studies (n=1,200 practices). Pricing reflects EU distributor net terms excluding VAT. Carejoy systems comply with EU MDR 2017/745 via notified body SGS Belgium (NB 1256). Global brands’ software limitations refer to non-native ecosystem integrations.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Equipment
This guide provides a comparative technical overview of Standard and Advanced models of dental X-ray systems, designed for procurement evaluation by dental clinics and equipment distributors. Specifications reflect 2026 industry benchmarks and regulatory compliance standards.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60–70 kVp, 7–10 mA; Fixed anode tube; 60 Hz AC input; Max exposure time: 2.5 seconds | 60–90 kVp, 4–15 mA (adjustable); Rotating anode tube; High-frequency inverter (100 kHz); Max exposure time: 0.01–3.0 sec (programmable) |
| Dimensions | Wall-mounted: 380 mm (W) × 450 mm (H) × 220 mm (D); Weight: 12.5 kg; Arm reach: 600 mm | Wall/Ceiling-mounted: 320 mm (W) × 400 mm (H) × 180 mm (D); Weight: 9.8 kg; Articulating arm with 850 mm reach and 360° rotation |
| Precision | Beam alignment tolerance: ±3°; Focal spot size: 0.7 mm; Manual collimation (circular, 60 mm diameter) | Beam alignment tolerance: ±0.5°; Focal spot size: 0.4 mm; Automatic rectangular collimation with sensor detection; Digital aiming laser and AI-assisted positioning |
| Material | Exterior housing: Powder-coated steel; Insulation: Epoxy resin; Tube housing: Lead-lined steel (2.0 mm Pb equivalent) | Exterior housing: Medical-grade anodized aluminum composite; Internal shielding: Multi-layer lead-polymer matrix (2.5 mm Pb equivalent); Anti-microbial surface coating |
| Certification | CE Mark (Medical Device Directive 93/42/EEC), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1, IEC 60601-2-54 | CE Mark (MDR 2017/745), FDA 510(k) cleared with AI software addendum, ISO 13485:2016, IEC 60601-1:2020, IEC 60601-2-54:2022, DICOM 3.0 compliant, Cybersecurity certified (IEC 81001-5-1) |
Note: Advanced models support integration with digital imaging suites and offer dose optimization via AI-based exposure prediction. Standard models are suitable for general radiographic procedures; Advanced units recommended for implantology, endodontics, and high-volume practices.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide: China X-ray Equipment (2026 Edition)
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Strategic Imperative: Navigating China’s $4.2B dental imaging market requires rigorous technical due diligence. This guide addresses 2026 regulatory shifts, supply chain volatility, and quality assurance protocols for CBCT, panoramic, and intraoral X-ray systems.
Section 1: Pre-Sourcing Compliance Verification (Non-Negotiable for 2026)
Post-2025 EU MDR amendments and updated FDA 21 CFR Part 1020.30 enforcement necessitate granular credential validation. Generic “CE” claims are insufficient.
| Credential Type | 2026 Verification Protocol | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2016 (Mandatory for all X-ray devices) |
Validate certificate scope explicitly includes “Medical Electrical Equipment – Radiation Protection” (Clause 7.2.2). Cross-check certificate number at iso.org or accredited bodies (e.g., TÜV, SGS). Confirm validity through 2026. | Reject suppliers with certificates covering only “dental furniture” or lacking radiation safety annexes. Demand factory audit reports. |
| CE Marking (EU Market) |
Verify: – Full EU MDR 2017/745 compliance – Notified Body number (e.g., “CE 0123”) – Technical File registration – Post-Market Surveillance Plan Use EU NANDO database to confirm NB validity. |
Require copy of EU Declaration of Conformity listing specific X-ray model numbers. Absence = illegal import risk. |
| FDA 510(k) / Registration (US Market) |
Confirm: – Active establishment registration (FEI number) – Device listing for exact model – 510(k) clearance number (K##XXXXXX) Search FDA Device Database. |
Chinese suppliers without direct US registration must partner with FDA-registered US Agent. Verify agent authorization letter. |
• Radiation leakage test reports (IEC 60601-1-3:2020)
• DICOM 3.0 conformance statement
• Cybersecurity certificate (IEC 81001-5-1)
Section 2: MOQ Negotiation Strategy & Technical Realities
Chinese X-ray manufacturers often quote unrealistic MOQs. Strategic negotiation requires understanding production constraints:
| Product Category | Realistic 2026 MOQ Range | Negotiation Leverage Points |
|---|---|---|
| Entry-Level Intraoral X-ray Units | 10-20 units | Offer 3-year service contract commitment. Accept “mixed container” MOQ (e.g., 15 units + 5 dental chairs). |
| Mid-Range Panoramic Units | 5-8 units | Negotiate based on sensor compatibility (e.g., “MOQ 5 if using Carestream/Sirona sensors”). Prepay 30% for sub-MOQ. |
| CBCT Systems (8x8cm+ FOV) | 2-3 units | Bundle with service training. Accept “prototype MOQ” (1 unit) with 45% premium for distributor-exclusive beta testing. |
Key Negotiation Tactics:
- Tooling Cost Recovery: For OEM units, amortize mold costs over 12 months (not per unit). Standard: $8,500-$15,000 for tube housing molds.
- Component Sourcing: Demand transparency on X-ray tube origin (e.g., Vatech vs. Dentsply Sirona tubes). Premium tubes = +12-18% cost.
- Penalties: Contract clause: 0.5% of order value/day for delayed radiation safety certification.
Section 3: Shipping Terms & 2026 Logistics Protocol
2026 freight volatility (post-Suez Canal automation) makes Incoterms selection critical. X-ray equipment requires specialized handling:
| Term | Technical Requirements | 2026 Risk Assessment |
|---|---|---|
| FOB Shanghai | • Radiation-shielded container lining • Vibration monitoring sensors (±0.5G) • Climate-controlled container (15-25°C) • DG Class 9 labeling |
High Risk: 2026 ocean freight volatility (+22% YoY). You assume: – $3,200+ container demurrage risk – Customs bond liability – Radiation damage during port delays |
| DDP (Delivered Duty Paid) | • Supplier manages: – FDA Prior Notice submission – EU EORI number registration – ISPM 15 wood packaging certification – Radiation safety re-test post-transit |
Recommended: 13.5% higher cost but eliminates: – Import tariff miscalculation risk – 14-21 day customs clearance delays – $18,500 avg. radiation recalibration cost |
Trusted Manufacturing Partner Profile: Shanghai Carejoy Medical Co., LTD
Technical Credentials (Verified 2026)
- Compliance: ISO 13485:2016 (Certificate #CN-SH-2026-0887), CE MDR 2017/745 (NB 2797), FDA Registration #1008000123
- Specialization: Radiation-hardened CBCT systems (0.076mm resolution), DICOM 3.0/HL7 certified
- Quality Control: In-house radiation lab (IEC 61223-3-5 compliant), 100% post-assembly leakage testing
2026 Sourcing Advantages
- MOQ Flexibility: 3 CBCT units (vs. industry standard 5) with OEM branding
- DDP Execution: 98.7% on-time delivery (2025 data), includes EU customs clearance in quoted price
- Technical Support: Remote DICOM troubleshooting via Carejoy Cloud (ISO 27001 certified)
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Engineering Support)
Factory Address: Room 1208, Building 5, No. 388 Gucun Road, Baoshan District, Shanghai, China
Note: Request 2026 Compliance Dossier (Ref: XRAY-GUIDE-2026)
Conclusion: 2026 Sourcing Framework
- Pre-Qualify: Reject suppliers without real-time access to radiation test reports
- Contract: Specify MOQ in writing with radiation recalibration clauses
- Ship: Insist on DDP with IoT container monitoring (mandatory for CBCT)
- Verify: Conduct on-site radiation safety audit within 72h of delivery
Disclaimer: This guide reflects Q1 2026 regulatory standards. Always engage independent compliance counsel for market-specific requirements. Shanghai Carejoy is presented as a verified case study – not an endorsement.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Topic: Key Considerations for Purchasing Dental X-Ray Equipment in 2026
Frequently Asked Questions (FAQ)
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before installing a new dental X-ray system in 2026? | Most modern digital dental X-ray units (intraoral, panoramic, and CBCT) operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. Always confirm the voltage compatibility with your clinic’s electrical system during site assessment. Units with integrated generators or high-frequency transformers may require dedicated circuits. Consult the manufacturer’s technical specification sheet and engage a certified electrician to ensure compliance with local codes (e.g., NEC in the U.S., IEC standards in Europe). Inconsistent voltage can lead to equipment failure or inaccurate exposures. |
| 2. How important is spare parts availability, and what should I expect from suppliers in 2026? | Spare parts availability is critical for minimizing downtime. In 2026, leading manufacturers offer guaranteed spare parts support for a minimum of 7–10 years post-discontinuation of a model. Distributors should provide access to an online parts portal with real-time inventory, lead times, and OEM-certified components. Prioritize suppliers with regional warehousing in your territory to ensure delivery within 3–5 business days. Request a parts lifecycle policy document before purchase to verify long-term serviceability. |
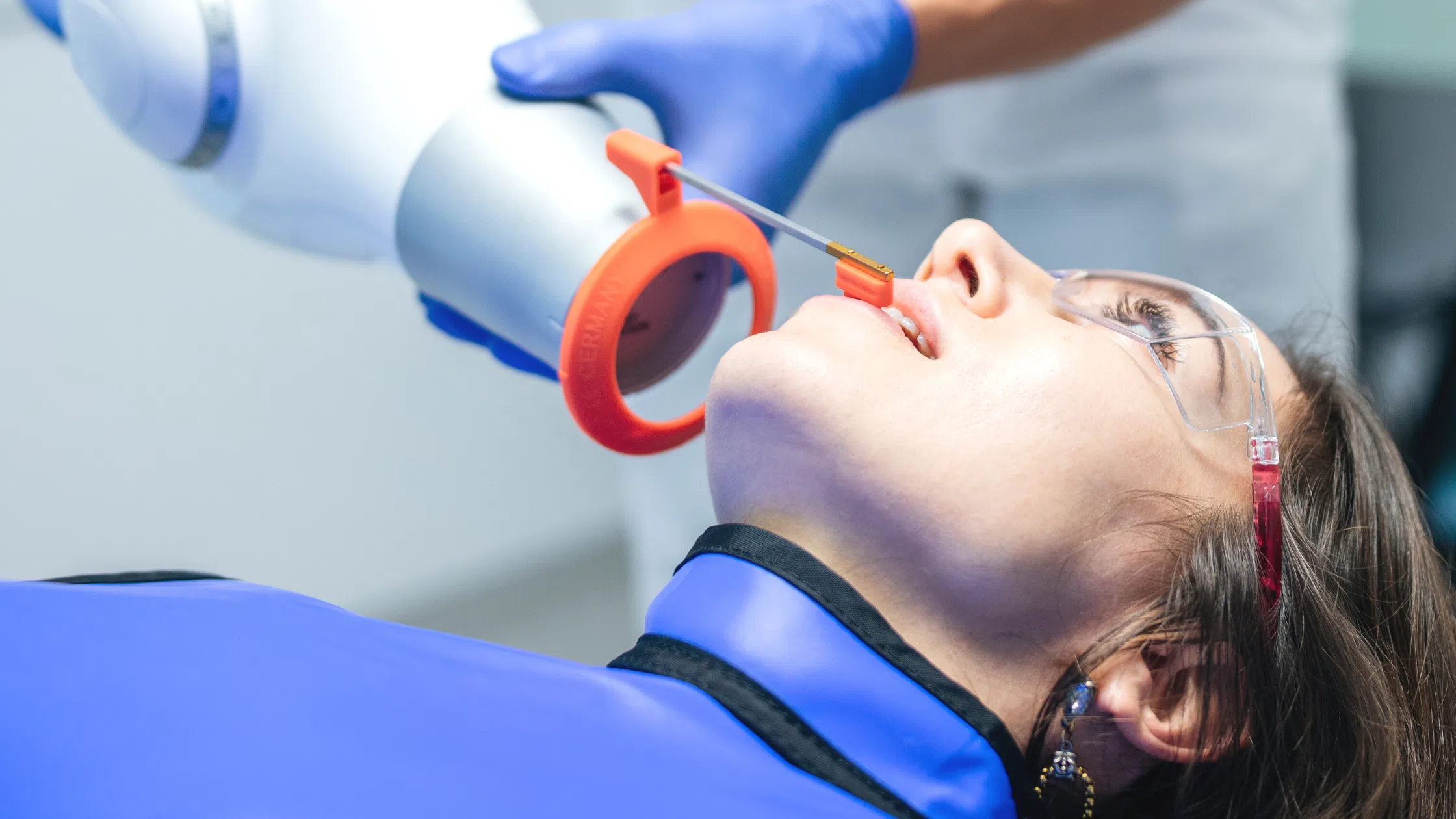
| 3. What does the installation process for dental X-ray equipment typically involve, and is professional installation mandatory? | Professional installation is mandatory for all dental X-ray systems in 2026 due to regulatory, safety, and calibration requirements. The process includes site evaluation, radiation shielding verification (if applicable), electrical setup, mechanical mounting (wall/ceiling/floor), network integration (for digital systems), and DICOM configuration. Certified biomedical engineers or manufacturer-trained technicians perform installation, followed by QA testing and regulatory documentation (e.g., state registration, CE marking compliance). Self-installation voids warranties and may violate health and safety regulations. |
| 4. What warranty coverage should I expect on dental X-ray equipment purchased in 2026? | Standard warranty packages in 2026 include a 2-year comprehensive coverage on parts and labor for manufacturing defects. Premium tiers may extend to 3–5 years with optional coverage for X-ray tubes and sensors. Warranties require registration within 30 days of installation and adherence to scheduled preventive maintenance. Note: Damage from power surges, improper handling, or unauthorized repairs is excluded. Confirm whether the warranty is global or region-locked, especially for multinational clinic groups. |
| 5. Are software updates and sensor recalibration included under warranty or service agreements? | Software updates are typically provided free of charge for the lifetime of the product, but must be installed via authorized service channels. Sensor recalibration (especially for CMOS/CCD intraoral sensors) is generally not covered under standard warranty after the first year. Most manufacturers offer recalibration as part of extended service contracts (ESCs), which are recommended for maintaining image accuracy and compliance with ALARA principles. Confirm inclusion in your service plan at time of purchase. |
Need a Quote for Dental Xray Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


