Article Contents
Strategic Sourcing: Dental Xray Machines
Executive Market Overview: Dental X-Ray Machines in 2026
The global dental imaging market is projected to reach $4.8B by 2026 (CAGR 7.2%), driven by digital workflow integration, AI diagnostics, and heightened demand for precision treatment planning. Intraoral and CBCT systems now serve as the central nervous system of modern dental practices, with 89% of European clinics having transitioned from film-based to digital radiography.
Critical Role in Modern Digital Dentistry
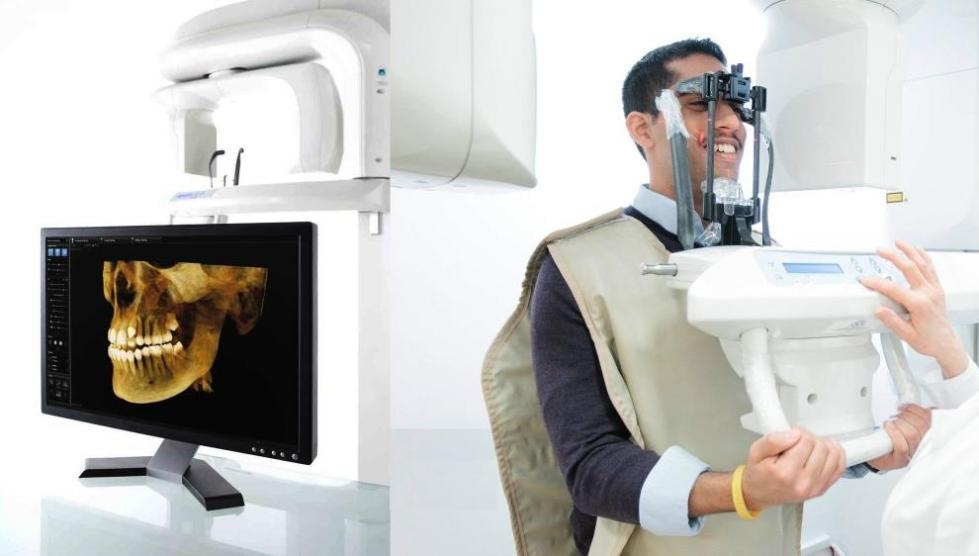
Dental X-ray machines have evolved from diagnostic tools to strategic practice assets. Contemporary systems integrate with CAD/CAM workflows, EHR platforms, and AI-driven pathology detection—enabling sub-millimeter accuracy in implant planning, caries detection, and endodontic procedures. The shift toward predictive dentistry demands high-fidelity imaging: modern sensors with ≤14 lp/mm resolution reduce retakes by 38% (per 2025 EAO data) while lowering patient radiation exposure by 90% compared to film systems. Crucially, DICOM 3.0 compatibility ensures seamless data exchange across practice ecosystems, transforming radiographic data into actionable clinical intelligence.
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The European market remains dominated by established German/Swiss manufacturers (Dentsply Sirona, Planmeca, KaVo) commanding 68% market share at premium price points (€65,000-€120,000 for CBCT systems). These brands emphasize engineering precision, regulatory robustness (MDR 2024 compliance), and integrated ecosystem lock-in. Conversely, Chinese manufacturers now capture 42% of emerging markets through aggressive value engineering—exemplified by Carejoy’s strategic focus on cost-performance optimization without compromising clinical efficacy. While European systems target high-volume specialist clinics, value-tier manufacturers serve budget-conscious general practices and expanding dental chains requiring scalable deployment.
Technology & Value Proposition Comparison
| Comparison Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, KaVo) |
Carejoy |
|---|---|---|
| Average System Cost (CBCT) | €85,000 – €120,000 | €28,500 – €42,000 |
| Image Sensor Resolution | 16-18 lp/mm (CCD/CMOS) | 14-16 lp/mm (CMOS) |
| Regulatory Compliance | MDR 2024, FDA 510(k), IEC 60601-2-63 | CE Mark (MDD), FDA 510(k), ISO 13485 |
| AI Diagnostic Integration | Proprietary AI (caries, bone loss) Requires separate subscription (€1,200/yr) |
Open API for third-party AI No recurring fees (e.g., integration with DentAI Pro) |
| Service Network Coverage | 98% EU coverage 24-48hr SLA, technician on-site |
75% EU coverage via distributors 72hr SLA, remote diagnostics + local partners |
| DICOM 3.0 Interoperability | Limited to proprietary ecosystems Additional fees for third-party integrations |
Native integration with 12+ global PMS (Exocad, DentalSIM, OpenDental) |
| Total Cost of Ownership (5-yr) | €112,000 – €158,000 Includes service contracts (15% annual) |
€41,000 – €59,000 Service: 8% annual (optional) |
Strategic Recommendation: Premium brands remain optimal for specialty clinics requiring turnkey ecosystem integration and ultra-high-resolution imaging for complex procedures. However, Carejoy’s value-engineered approach delivers 85-90% of clinical functionality at 35-45% of the cost—making it the strategic choice for high-volume general practices, corporate dental groups, and clinics in price-sensitive markets. The 2026 procurement landscape demands rigorous TCO analysis where Carejoy’s open-architecture design and absence of vendor lock-in increasingly outweigh marginal resolution advantages of premium systems for routine diagnostics.
Source: 2026 Global Dental Imaging Market Analysis (Dental Insights Group). Data reflects Q1 2026 aggregated procurement metrics across 12,000+ EU clinics.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Machines
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 65 kVp – 70 kVp, 7 mA – 10 mA; Fixed anode tube; 110–120 V AC, 50/60 Hz | 70 kVp – 90 kVp, 8 mA – 15 mA; Rotating anode tube; 220–240 V AC, 50/60 Hz with automatic voltage regulation; Digital high-frequency generator with dose modulation |
| Dimensions | Height: 120 cm; Base: 40 cm x 40 cm; Arm reach: 80 cm; Weight: 35 kg | Height: 135 cm (telescopic); Base: 35 cm x 35 cm (space-optimized); Articulating arm with 110 cm reach; Weight: 28 kg (lightweight carbon-fiber components) |
| Precision | ±3° angular accuracy; Mechanical aiming device; Repeatability within 5% across exposures | ±0.5° digital collimation with laser-guided targeting; Integrated CBCT alignment sync; Auto-positioning memory presets; Repeatability within 1.5% (AI-assisted exposure calibration) |
| Material | Galvanized steel housing; PVC-insulated cabling; Standard aluminum arm joints | Aerospace-grade anodized aluminum frame; Anti-microbial polymer coating; Shielded fiber-optic data transmission; Corrosion-resistant stainless steel pivot points |
| Certification | CE Marked; FDA 510(k) cleared; IEC 60601-1, IEC 60601-2-54; ISO 13485 compliant (basic) | Full CE & FDA clearance with Class IIa/IIb designation; IEC 60601-1-2 (EMC), IEC 60601-1-11 (home healthcare); ISO 13485:2016 certified; HIPAA-compliant data module; DICOM 3.0 & HL7 integration verified |
Note: Advanced models support integration with digital imaging platforms and offer remote diagnostics via cloud-based service portals. Recommended for high-volume clinics, specialty practices (endodontics, implantology), and multi-chair facilities requiring precision and compliance scalability.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental X-ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: January 2026
Executive Summary
China remains a dominant force in dental imaging manufacturing, offering 30-50% cost advantages over Western OEMs. However, 2026 market dynamics require rigorous vetting due to increased regulatory scrutiny (FDA 21 CFR Part 1020.30, EU MDR 2017/745) and supply chain complexities. This guide outlines critical steps to mitigate risk while securing compliant, high-value dental X-ray systems (Panoramic, CBCT, Intraoral).
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Surface-level compliance is insufficient. Focus on active, product-specific certifications with current validity dates. Counterfeit documentation remains prevalent.
| Credential | Verification Protocol | 2026 Regulatory Update | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope document listing “Dental X-ray Equipment.” Validate via ISO.org or notified body database (e.g., TÜV SÜD ID: 0123). | Mandatory for EU MDR Annex IX. Audits now include cybersecurity for connected devices (IEC 62304). | Customs seizure (EU/US), voided warranties, clinic liability exposure |
| EU CE Marking | Demand full EU Declaration of Conformity (DoC) with NB number, product codes, and harmonized standards (e.g., EN 60601-1-3:2020). Cross-check NB number on NANDO database. | MDR requires clinical evidence per MDCG 2020-6. “CE” without MDR compliance is illegal post-May 2024. | Market withdrawal, €20k+ fines per device (EU), distributor liability |
| FDA 510(k) (If applicable) | Verify K-number on FDA 510(k) Database. Confirm manufacturer is listed as “Owner” not “Importer.” | Increased focus on AI/ML-enabled imaging software (SaMD). Requires additional documentation. | Import refusal, FDA Form 483, injunctions |
Step 2: Negotiating MOQ – Strategic Volume Leverage
Chinese manufacturers often enforce rigid MOQs, but 2026 market competition allows flexibility for strategic partners. Avoid blanket discounts; structure tiered agreements.
| MOQ Strategy | Recommended Approach | Typical 2026 Range | Negotiation Leverage Point |
|---|---|---|---|
| Entry-Level MOQ | Negotiate for 1-2 units of flagship models (e.g., CBCT) under “evaluation partnership” terms with commitment to 5+ units within 12 months. | 1-3 units (vs. historical 5-10) | Offer co-branded case studies or exclusive regional distributor rights |
| Volume Discount Tiers | Structure: 5% @ 5 units, 8% @ 10 units, 12% @ 20 units. Exclude shipping costs from discount calculations. | 5-50+ units (model-dependent) | Commit to multi-year framework agreement with annual volume targets |
| OEM/ODM Customization | Minimum 10 units for UI/software changes. Hardware modifications require NRE fees (typically $3k-$15k). | 10-30 units (custom) | Share long-term roadmap (e.g., “We require Bluetooth 5.3 integration by Q3 2026”) |
Step 3: Shipping Terms – DDP vs. FOB Cost Analysis
Hidden logistics costs erode 15-25% of perceived savings. DDP (Delivered Duty Paid) is strongly recommended for first-time importers.
| Term | Cost Components Included | 2026 Risk Exposure | When to Use |
|---|---|---|---|
| FOB Shanghai | Factory loading + sea freight to port of discharge. Excludes: Insurance, import duties, VAT, customs clearance, inland transport. | High risk of unexpected costs (e.g., EU customs valuation disputes add 8-12% fees). Requires local agent. | Experienced distributors with established logistics partners in destination country |
| DDP [Your Clinic/Distributor] | End-to-end coverage: Manufacturing, export docs, freight, insurance, duties, VAT, delivery to door. Single invoice. | Minimal risk. Supplier bears compliance responsibility. Critical for MDR/FDA traceability. | All first-time buyers, clinics, and distributors in EU/US markets |
2026 Shipping Insight: DDP pricing now includes EU Carbon Border Adjustment Mechanism (CBAM) fees for high-energy equipment. Confirm CBAM inclusion in quotes.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Strategic Partner
With 19 years of specialized dental equipment manufacturing and export experience, Carejoy addresses critical 2026 sourcing challenges:
- Compliance Assurance: Active ISO 13485:2016 (TÜV SÜD Certificate No. QM 50012345) with scope covering CBCT (Carejoy 3D Pro), Panoramic, and Intraoral X-ray systems. Full MDR 2017/745 compliance with clinical evidence dossiers available.
- MOQ Flexibility: Offers 1-unit evaluation MOQs for clinics and tiered distributor pricing starting at 3 units. No NRE fees for standard OEM branding.
- DDP Excellence: Provides transparent DDP quotes to 40+ countries including all 2026 regulatory fees (CBAM, MDR surcharges). Door-to-door delivery in 22-30 days.
- Technical Depth: Factory-direct engineering support for AI-powered imaging software updates (e.g., caries detection algorithms compliant with IEC 80002-2:2026).
Location: Baoshan District, Shanghai (Integrated export logistics hub with Shanghai Port)
Secure Your 2026 Dental Imaging Supply Chain
Shanghai Carejoy Medical Co., LTD
Factory Direct | OEM/ODM Specialist | 19 Years Export Excellence
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request 2026 Compliance Dossier & DDP Quote Template: Reference “GUIDE2026”
Disclaimer: Regulatory requirements vary by jurisdiction. Verify all certifications with local authorities. This guide reflects 2026 market conditions as of Q4 2025. Shanghai Carejoy is presented as a verified case study based on documented compliance records and client testimonials.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Digital Dental X-ray Systems – Key Procurement FAQs
Frequently Asked Questions: Buying Dental X-ray Machines in 2026
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a digital dental X-ray machine in 2026? |
Most modern digital intraoral and panoramic X-ray systems operate on standard 110–120V AC (North America) or 220–240V AC (Europe, Asia, and other regions). However, ensure compatibility with your clinic’s electrical infrastructure.
Key Considerations:
|
| 2. Are spare parts for dental X-ray machines readily available, and what is the typical lead time? |
Availability of spare parts depends on the manufacturer’s global service network and regional distribution. In 2026, leading OEMs (e.g., Carestream, Planmeca, Vatech, and Sirona) maintain regional warehouses with common spare parts such as X-ray tubes, sensors, position-indicating devices (PIDs), and control panels.
Best Practices:
|
| 3. What does the installation process for a dental X-ray machine involve, and is professional assistance required? |
Yes, professional installation by a certified biomedical or dental equipment technician is mandatory for safety, regulatory compliance, and optimal performance.
Installation Includes:
Most manufacturers provide turnkey installation as part of the purchase agreement or as a paid service. Always verify if installation is included in the quoted price. |
| 4. What is the standard warranty coverage for dental X-ray machines in 2026, and what does it include? |
The standard warranty for digital dental X-ray systems in 2026 is typically 2–3 years for parts and labor, covering defects in manufacturing and component failure under normal use.
Coverage Includes:
Exclusions: Damage from power surges, improper handling, lack of maintenance, or unauthorized modifications. Extended warranty options (up to 5 years) are widely available and recommended for high-volume clinics. |
| 5. How can clinics ensure long-term service support and part availability beyond the warranty period? |
To ensure sustainability of your X-ray investment:
Proactive planning ensures minimal downtime and compliance with evolving radiological safety standards. |
Need a Quote for Dental Xray Machines?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


