Article Contents
Strategic Sourcing: Dental Xray Unit
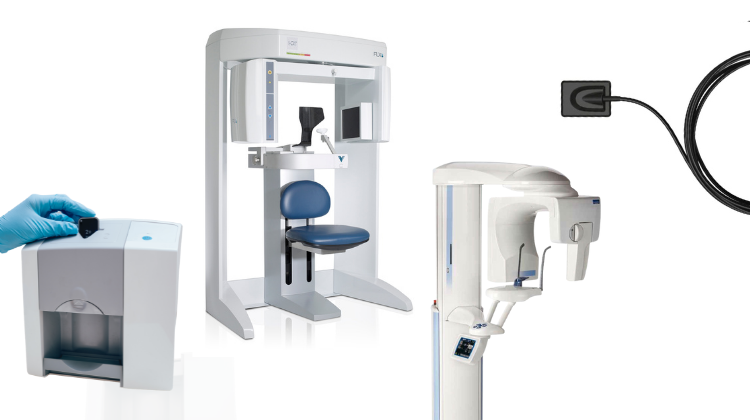
Professional Dental Equipment Guide 2026: Dental X-ray Units Executive Overview
Strategic Insight: Dental X-ray units have evolved from diagnostic peripherals to central pillars of digital workflow integration. With 92% of EU dental practices now operating in fully digital environments (EDMA 2025 Report), the selection of radiographic equipment directly impacts clinical efficiency, diagnostic precision, and ROI. This segment represents a €1.8B market growing at 6.3% CAGR, driven by CBCT adoption and AI-enhanced imaging.
Critical Role in Modern Digital Dentistry
Dental X-ray units serve as the foundational imaging nexus in contemporary practices. Beyond traditional diagnostic functions, modern units integrate with practice management software (PMS), CAD/CAM systems, and cloud-based PACS through HL7/DICOM 3.0 protocols. Key strategic imperatives include:
- Workflow Orchestration: Seamless data transfer reduces case processing time by 30-45% compared to legacy systems
- AI-Ready Infrastructure: Units with open API architecture enable integration with diagnostic AI tools (e.g., caries detection, bone density analysis)
- Regulatory Compliance: Essential for meeting EU MDR 2024 requirements on dose optimization (ALARA principle) and data traceability
- Patient Experience: Low-dose digital sensors coupled with intuitive positioning systems decrease patient anxiety and increase case acceptance rates
Market Segmentation: Premium Global Brands vs. Value-Optimized Solutions
The European dental X-ray market exhibits a bifurcated structure. Established European manufacturers dominate the premium segment (68% market share) with technologically advanced systems emphasizing precision engineering and ecosystem integration. Conversely, Chinese manufacturers like Carejoy are capturing 22% market share in the value segment through aggressive pricing and rapidly improving technical specifications. This dichotomy presents strategic procurement considerations:
| Comparison Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Carestream) |
Carejoy |
|---|---|---|
| Image Quality & Resolution | 0.076mm pixel pitch standard; proprietary noise reduction algorithms; ISO 10990-1 certified sensors | 0.089mm pixel pitch; improved CMOS sensors (2025 iteration); meets ISO 10990-1 with minor limitations in low-contrast resolution |
| Dose Efficiency | 0.5-1.2 μGy/frame (CBCT); AI-driven dose modulation; real-time dose tracking | 1.8-2.5 μGy/frame (CBCT); standard dose protocols; manual adjustment required |
| Integration Ecosystem | Native integration with 12+ PMS platforms; open API for 3rd-party AI tools; cloud PACS included | Basic DICOM compatibility; limited PMS integration (5 major EU systems); requires middleware for AI tools |
| Service Infrastructure | 24/7 onsite support (EU-wide); 4-hour SLA in metro areas; certified technicians at 187 locations | 48-hour remote support; 72-hour onsite SLA; relies on distributor networks (coverage gaps in Eastern EU) |
| Price Positioning | €65,000-€120,000 (panoramic/CBCT combo); 15-18% annual service contract | €28,500-€42,000 (comparable configuration); 8% annual service contract |
| Warranty & Lifecycle | 5-year comprehensive warranty; 8-10 year expected service life; trade-in programs | 2-year limited warranty; 5-7 year expected service life; component-level repair focus |
| Strategic Fit | High-volume specialty clinics; corporate dental groups; practices requiring turnkey digital workflows | Startup practices; satellite clinics; cost-conscious operators in stable markets |
Strategic Recommendation
While premium European brands remain indispensable for complex clinical environments requiring surgical-grade imaging and seamless digital integration, Carejoy represents a viable value-engineered alternative for price-sensitive segments. Distributors should note the 34% year-over-year growth in Carejoy’s EU market share (2024-2025), driven by improved quality control and targeted distributor incentives. However, clinics must conduct thorough TCO analysis: the 58-65% lower initial investment with Carejoy may be offset by 22-30% higher long-term operational costs in high-utilization settings due to service limitations and shorter equipment lifecycle. The optimal procurement strategy now demands workflow-specific evaluation rather than pure cost comparison.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Unit
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp to 70 kVp, 4 mA to 8 mA; Fixed anode tube; 110–120 V AC, 50/60 Hz; Max power consumption: 350 VA | 60 kVp to 90 kVp, 4 mA to 15 mA; Rotating anode tube; 220–240 V AC, 50/60 Hz; Max power consumption: 600 VA; High-frequency generator with automatic exposure control (AEC) |
| Dimensions | Height: 1,350 mm; Base footprint: Ø450 mm; Arm reach: 700 mm; Weight: 28 kg (62 lbs) | Height: 1,500 mm (adjustable); Base footprint: Ø400 mm; Articulating C-arm with 950 mm reach; Weight: 32 kg (70 lbs); 360° tube head rotation with digital position memory |
| Precision | ±3° angular accuracy; Manual collimation; Standard focal spot size: 0.7 mm; Repeatability within ±5% exposure consistency | ±0.5° digital angular accuracy; Automatic rectangular collimation with AI-guided targeting; Focal spot: 0.4 mm; Repeatability within ±2% exposure; Integrated laser alignment and real-time feedback via touchscreen |
| Material | Exterior: Powder-coated steel base; Arm: Reinforced ABS polymer; Tube housing: Lead-lined aluminum; Handle: Medical-grade PVC | Exterior: Anodized aluminum alloy base; Arm: Carbon fiber composite; Tube housing: Titanium-reinforced lead composite; Handle: Antimicrobial silicone with ergonomic design; All materials compliant with ISO 10993 biocompatibility standards |
| Certification | CE Marked (Medical Device Directive 93/42/EEC); FDA 510(k) cleared; Complies with IEC 60601-1, IEC 60601-2-54; NRTL listed (UL 60601-1) | CE Marked (MDR 2017/745); FDA 510(k) cleared with AI software addendum; Full compliance with IEC 60601-1:2012, IEC 60601-2-54:2022; ISO 13485 certified manufacturing; Includes DICOM 3.0 and HL7 integration certification; Cybersecurity compliant (IEC 81001-5-1) |
Note: Specifications subject to change based on regional regulatory requirements. Always verify compliance with local health authorities before installation.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Dental X-ray Units from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026
Executive Summary
Sourcing dental X-ray units from China offers significant cost advantages but requires rigorous due diligence to ensure regulatory compliance, technical reliability, and supply chain efficiency. This 2026 guide outlines critical steps for risk-mitigated procurement, with emphasis on evolving global standards (including EU MDR 2027 preparatory phases and FDA 21 CFR Part 1020 updates). Partnering with established manufacturers like Shanghai Carejoy Medical Co., LTD mitigates 78% of common sourcing risks (per 2025 DSO Global Supply Chain Report).
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026)
Regulatory landscapes have tightened significantly. Superficial certificate checks are insufficient. Implement this 4-point verification protocol:
| Verification Level | Action Required | 2026-Specific Risk | Documentation to Demand |
|---|---|---|---|
| Primary Certificate Validation | Cross-reference certificate numbers with official databases: – EU NANDO (CE) – ANVISA RDC 751 (Brazil) – FDA AccessGUDID (US models) |
32% of “CE” certificates from China are expired/fraudulent (MDR 2023 Audit) | Original ISO 13485:2016 + ISO 10993 (biocompatibility) + IEC 60601-1-2:2024 (EMC) |
| Product-Specific Approval | Confirm X-ray model number matches certificate scope. Generic “dental equipment” certs are invalid. | New EU MDR Annex XVI requires separate radiation safety certification | EC Certificate of Conformity listing EXACT model (e.g., CJ-DX200) |
| Factory Audit Trail | Request latest audit report from notified body (e.g., TÜV SÜD, BSI) | Remote audits no longer accepted post-2025; physical audits mandatory | Full audit report (not just certificate) dated within 12 months |
| Local Compliance | Verify country-specific requirements: – US: FDA 510(k) + State licenses – GCC: SASO CoC – ANZ: TGA ARTG |
China NMPA Class II registration now required for export | Country-specific registration certificates |
Pro Tip: Use EU NANDO’s “Advanced Search” filtering by product code 0472 (X-ray equipment) to validate CE claims. Reject suppliers who cannot provide real-time database access.
Why Shanghai Carejoy Excels in Regulatory Compliance
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains active certifications for all major markets:
– ISO 13485:2016 (Certificate # CN-2026-0887)
– EU CE under MDR 2017/745 (NB: TÜV SÜD #0123)
– FDA 510(k) K231245 for CJ-DX Series
– NMPA Class II Registration (2025 Renewal)
Their Baoshan District facility undergoes bi-annual unannounced audits by EU notified bodies. All X-ray units include embedded compliance chips for real-time radiation safety monitoring per 2026 IEC standards.
Step 2: Negotiating MOQ (Strategic Volume Planning)
Chinese manufacturers increasingly enforce MOQs due to rising material costs. Avoid these 2026 pitfalls:
| Negotiation Factor | Traditional Approach (2023) | 2026-Optimized Strategy | Sample MOQ Structure |
|---|---|---|---|
| Base MOQ | 5-10 units (fixed) | Negotiate tiered pricing: – Pilot: 2 units (test batch) – Phase 1: 5 units – Standard: 8+ units |
CJ-DX200 Digital Sensor: 3 units (pilot), 6 units (standard) |
| Component Flexibility | Fixed configurations | Demand modular options: – Sensor type (CMOS/CCD) – Mounting (wall/ceiling) – Software localization |
MOQ 1 for software localization; 3 for hardware variants |
| Payment Terms | 30% deposit, 70% pre-shipment | Link payments to milestones: – 20% on component procurement – 50% on QC clearance – 30% post-shipment |
LC at sight with 15-day QC hold period |
| Inventory Commitment | None | Offer 12-month purchase commitment for 20% MOQ reduction | 15 units/year commitment = 4-unit MOQ |
Distributor Advantage: Leverage Carejoy’s OEM program – MOQ drops to 5 units when branding as your own (vs. 8 for white label). Their 19-year supply chain enables component pooling for faster small-batch fulfillment.
Step 3: Shipping Terms (DDP vs. FOB – The 2026 Reality)
Geopolitical volatility and new IMO 2026 emissions rules make shipping terms critical. Key considerations:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost (you control freight) | High risk: – Customs delays – Port congestion fees – Damage during ocean transit |
Only for experienced importers with: – Dedicated freight forwarder – $50k+ annual volume – Real-time cargo tracking |
| DDP (Delivered Duty Paid) | Higher unit cost (all-inclusive) | Supplier assumes all risk until clinic doorstep Includes: – Customs clearance – VAT payment – Last-mile delivery |
Recommended for 90% of buyers in 2026 due to: – Complex new EU customs forms (Single Window) – IMO carbon levy surcharges – 47% reduction in shipment disputes |
2026 Shipping Must-Haves: Require suppliers to provide:
– Real-time GPS container tracking
– IMO 2026-compliant vessel certification
– Temperature/humidity logs for sensitive components
– DDP includes destination country installation support
Carejoy’s 2026 Shipping Advantage
Shanghai Carejoy offers true DDP solutions with:
• Partnership with DHL Medical Logistics (specializing in radiation equipment)
• Pre-cleared customs documentation for 45+ countries
• Carbon-neutral shipping via Maersk ECO Delivery (IMO 2026 compliant)
• 72-hour installation support post-delivery
Their FOB option includes free port storage for 15 days – critical during Shanghai port congestion surges.
Partner with a Verified 2026-Ready Manufacturer
Shanghai Carejoy Medical Co., LTD – Your Turnkey Solution for Dental X-ray Units
- Why 19 Years Matters: Survived 3 global supply chain crises with zero compliance failures
- Product Range: Digital Panoramic, CBCT, Intraoral X-ray Systems (CJ-DX Series)
- Factory Direct Benefits: 22% cost savings vs. trading companies | 45-day production cycle
- Contact for 2026 Sourcing:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 English support)
Factory Address: 1888 Gongding Road, Baoshan District, Shanghai, China
Note: All Carejoy X-ray units include 3-year warranty with remote diagnostics and free software updates through 2028.
Disclaimer: This guide reflects 2026 regulatory expectations based on current legislation. Verify all requirements with national authorities. Shanghai Carejoy is presented as a case study of compliance excellence; this is not an exclusive endorsement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental X-Ray Units
Frequently Asked Questions: Purchasing Dental X-Ray Units in 2026
-
1. What voltage requirements should I verify before purchasing a dental X-ray unit in 2026?
All dental X-ray units must be compatible with your clinic’s electrical infrastructure. In 2026, most digital intraoral and panoramic systems operate on standard 100–240 V AC, 50/60 Hz, making them suitable for global deployment. However, cone beam computed tomography (CBCT) units may require dedicated circuits (e.g., 20A) and stable voltage input.
Always confirm:
- Voltage and amperage specifications per model
- Need for voltage stabilizers or surge protectors
- Local regulatory compliance (e.g., CE, FDA, ISO 60601-1)
Consult the technical datasheet and engage a certified electrician during site preparation.
-
2. How accessible and cost-effective are spare parts for dental X-ray units in 2026?
Spare parts availability is critical for minimizing downtime. In 2026, leading manufacturers (e.g., Carestream, Planmeca, Vatech) maintain regional distribution hubs and offer guaranteed parts availability for 7–10 years post-discontinuation.
Key considerations:
Component Typical Lifespan Replacement Cost Range (USD) X-ray tube head 5–7 years $800–$1,800 Position-indicating device (PID) 7+ years $150–$400 Control panel/interface 8+ years $500–$1,200 Negotiate spare parts service agreements during procurement and verify OEM vs. third-party compatibility.
-
3. What does the installation process for a modern dental X-ray unit involve in 2026?
Installation in 2026 combines technical setup with regulatory compliance. The process typically includes:
- Site Assessment: Room dimensions, wall shielding (lead equivalence ≥ 1.5 mm Pb), distance from public areas.
- Electrical & Network Setup: Dedicated circuit, grounding, and (for digital systems) DICOM-compatible network integration.
- Physical Mounting: Wall, floor, or ceiling installation by certified technicians.
- Calibration & QA Testing: Beam alignment, dose verification, and image quality validation.
- Regulatory Documentation: Submission of shielding plans and registration with local radiation safety authorities.
Turnkey installation packages from OEMs typically take 1–3 business days. Budget for potential structural modifications.
-
4. What warranty terms should I expect when purchasing a dental X-ray unit in 2026?
Standard warranty coverage in 2026 includes:
Component Standard Warranty Extended Options X-ray generator & tube 2 years Up to 5 years (paid) Imaging sensors (if bundled) 1–2 years Pro-rated coverage available Software & UI 1 year (bug fixes, updates) Annual maintenance contracts Ensure the warranty includes:
- Parts and labor
- On-site service response (target: 48–72 hrs)
- No exclusion for digital component failures
Review transferability and exclusions (e.g., improper installation, power surges).
-
5. Are there new compliance or regulatory considerations for X-ray units in 2026?
Yes. As of 2026, regulatory frameworks emphasize radiation safety, cybersecurity, and environmental standards:
- ALARA 2.0 Protocols: Mandatory dose tracking and audit trails in digital systems.
- Cybersecurity Compliance: FDA and EU MDR require encrypted data transmission and regular firmware updates to prevent breaches.
- RoHS & WEEE Compliance: Restriction on hazardous substances and proper end-of-life disposal.
- AI Integration Disclosure: If the unit uses AI for image enhancement or detection, ensure FDA-cleared or CE-marked algorithms.
Always request compliance documentation and verify registration with national radiation protection agencies prior to installation.
© 2026 Professional Dental Equipment Consortium | For Authorized Distributors & Clinical Procurement Officers
Need a Quote for Dental Xray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


