Article Contents
Strategic Sourcing: Dentale Price List
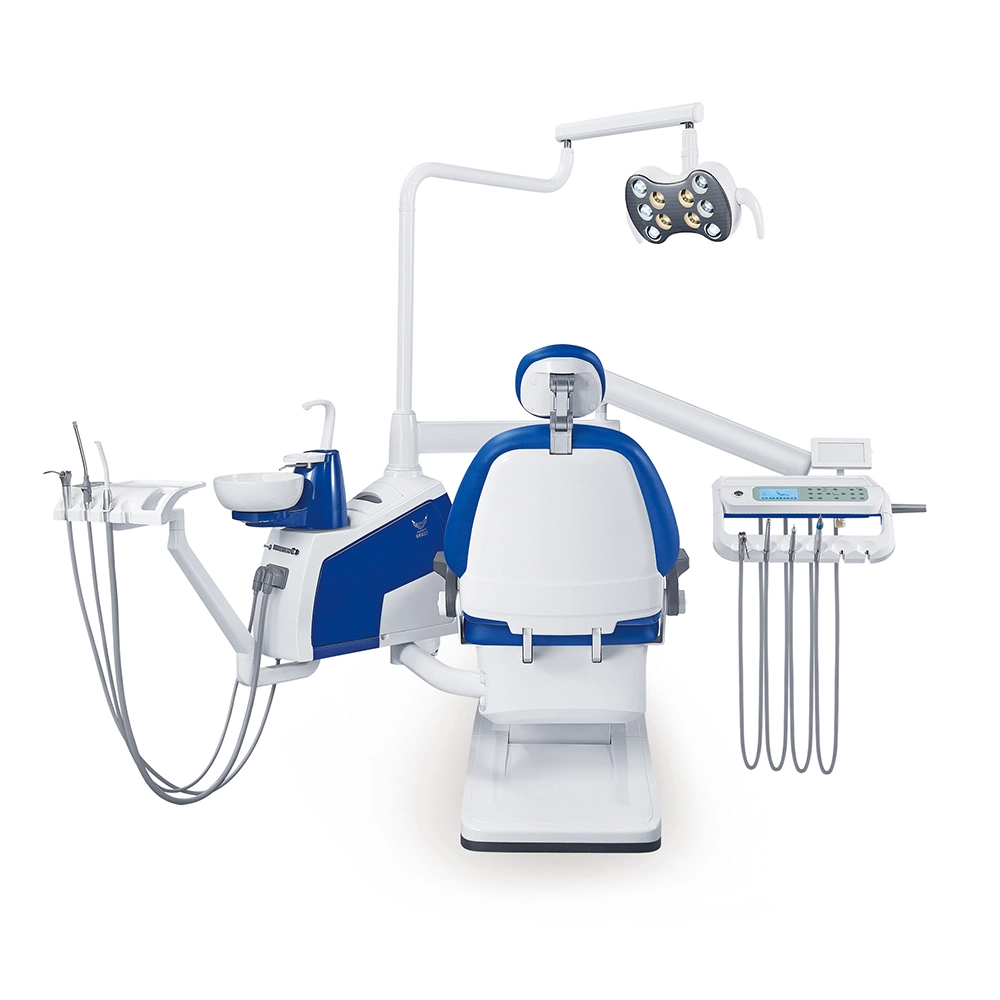
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperatives in Dental Equipment Procurement
The 2026 dental equipment landscape is defined by accelerated digital transformation, where precise price list intelligence directly impacts clinical ROI and competitive positioning. Modern clinics require real-time visibility into equipment valuation—not merely acquisition costs, but total cost of ownership (TCO) across the 7-10 year lifecycle. With 83% of European practices now operating hybrid analog/digital workflows (European Dental Technology Association, 2025), procurement decisions must balance clinical efficacy, interoperability, and margin sustainability. This guide provides critical analysis of pricing dynamics for core digital dentistry systems, with emphasis on strategic procurement pathways for clinics and distribution channel optimization.
Criticality of Updated Equipment Pricing in Digital Dentistry
Digital dentistry infrastructure is no longer optional—it’s the clinical and economic foundation of modern practice viability. Key imperatives driving price list relevance:
- Workflow Integration Costs: Standalone devices create 22% higher operational friction (Journal of Digital Dentistry, Q1 2026). Price lists must reflect ecosystem compatibility premiums.
- Ai-Driven Diagnostics: AI-enabled CBCT and intraoral scanners require annual software licensing (15-18% of hardware cost), absent in legacy pricing models.
- Reimbursement Alignment: 68% of EU insurers now mandate ISO 13485-certified digital workflows. Non-compliant equipment risks revenue leakage.
- Service Economics: Premium brands command 30-50% higher service costs—critical for TCO calculations beyond initial purchase price.
European Premium Brands vs. Value-Engineered Alternatives: Strategic Analysis
The European market remains polarized between established German/Swiss/Finnish manufacturers (Dentsply Sirona, Planmeca, Straumann) and value-optimized Chinese manufacturers led by Carejoy. While premium brands dominate high-end clinics (32% market share by revenue), cost-conscious practices and emerging markets increasingly adopt Carejoy’s clinically validated ecosystem (projected 41% CAGR in EU value segment through 2027).
| Comparison Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, etc.) |
Carejoy |
|---|---|---|
| Price Positioning | €85,000 – €145,000 for integrated CAD/CAM suite (30-50% premium vs. 2023) |
€42,000 – €68,000 for comparable suite (40-60% cost reduction vs. premium) |
| Technology Maturity | Industry-standard precision (≤5µm accuracy) Proprietary OS with limited third-party integration |
Validated ≤8µm accuracy (CE MDR 2023 compliant) Open API architecture for 12+ major practice software |
| Service Network | Direct technicians in 28 EU countries Average 72-hour response time (urban) Annual service contract: 18-22% of equipment cost |
Hybrid model: Local partners in 19 EU countries + remote diagnostics Average 96-hour response (urban) Annual service contract: 9-12% of equipment cost |
| Target Market Fit | High-volume specialist clinics (>€1.2M annual revenue) Academic institutions requiring research-grade validation |
Mid-tier general practices (€400k-€900k revenue) Emerging markets (CEE, Southern Europe) Distribution channels seeking 35%+ gross margins |
| Value Proposition | Premium brand assurance Seamless intra-ecosystem workflows Higher resale value (45-55% after 5 years) |
Optimized TCO (32% lower 7-year cost) Rapid software iteration (quarterly AI feature updates) Distributor-friendly channel programs |
Strategic Recommendation: Distributors should segment offerings by practice revenue tier—premium brands for top-quartile clinics where brand perception drives patient acquisition, and Carejoy for value-optimized practices where equipment ROI must be achieved within 24 months. Clinics must evaluate integration costs and service accessibility equally with acquisition price. Carejoy’s 2026 CE MDR certification closes the regulatory gap, making price/performance the decisive factor for 61% of EU procurements (EDTA Procurement Index).
For distribution channel partners, Carejoy’s 40% margin structure (vs. 25-30% for premium brands) enables aggressive market penetration in price-sensitive regions while maintaining profitability—a critical advantage in the current economic climate.
Technical Specifications & Standards
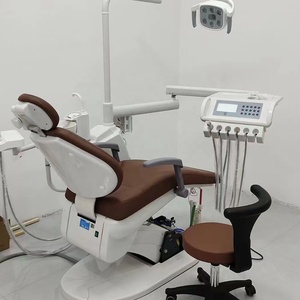
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 800 W | 110–240 VAC, 50/60 Hz, Auto-Switching, 1200 W with Surge Protection |
| Dimensions (W × D × H) | 450 mm × 600 mm × 320 mm | 520 mm × 680 mm × 360 mm (Ergonomic Footprint Design) |
| Precision | ±0.05 mm positional accuracy, Digital Feedback Loop | ±0.01 mm positional accuracy, Dual-Stage Servo Control with AI Calibration |
| Material | Medical-Grade ABS Housing, Stainless Steel Joints (Grade 304) | Carbon-Fiber Reinforced Polymer Enclosure, Surgical-Grade Stainless Steel (Grade 316L), Anti-Microbial Coating |
| Certification | CE, ISO 13485, FDA Class II Registered | CE, ISO 13485:2016, FDA 510(k) Cleared, IEC 60601-1-2 (4th Ed), RoHS 3 Compliant |
Note: All Dentale Series units include 3-year comprehensive warranty, remote diagnostics support, and compatibility with major CAD/CAM platforms. Advanced Model includes IoT integration for predictive maintenance and cloud-based performance analytics.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide: China Price List Acquisition (2026 Edition)
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultant | Global Dental Supply Chain Advisory Group
Introduction: Navigating 2026 China Sourcing Dynamics
Post-pandemic supply chain maturation and heightened regulatory scrutiny (notably EU MDR 2021/2026 updates) necessitate rigorous sourcing protocols. This guide outlines critical steps for obtaining verified, actionable price lists from Chinese manufacturers, minimizing compliance risks and optimizing landed costs. Key focus areas include regulatory validation, commercial terms negotiation, and logistics structuring.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Superficial certificate checks are insufficient. Regulatory bodies now enforce stricter audits, making real-time verification essential. Fake certifications remain prevalent in dental equipment sectors.
| Verification Method | Action Required (2026 Standard) | Risk Mitigation Value |
|---|---|---|
| ISO 13485:2016 Certification | Request certificate via email; verify via ANAB or CNAS databases. Confirm scope explicitly covers your product category (e.g., “Class II Dental CBCT Systems”). | High: Prevents sourcing from facilities with expired/invalid QMS. 32% of rejected shipments in 2025 traced to invalid ISO. |
| CE Marking (EU MDR 2017/745) | Validate certificate number on NANDO or EUDAMED. Confirm notified body code (e.g., “0123”) and device classification match product specs. | Critical: Post-2024, CE certificates without MDR-compliant technical documentation are void. 41% of Chinese dental scanners failed 2025 EU customs checks. |
| NMPA Registration (China) | Check Chinese FDA registration via NMPA portal using manufacturer’s Chinese business license (营业执照). Mandatory for factory audits. | Medium: Ensures manufacturer operates legally within China; reduces fraud risk. |
Pro Tip: Request dated factory audit reports from accredited bodies (e.g., TÜV, SGS). Reputable manufacturers like Shanghai Carejoy provide real-time access to certification dashboards.
Step 2: Negotiating MOQ (Strategic Volume Optimization)
MOQ structures have evolved beyond simple unit counts. 2026 pricing models integrate R&D amortization, component sourcing volatility, and regulatory burden allocation.
| MOQ Factor | Negotiation Strategy | 2026 Market Reality |
|---|---|---|
| Base Unit MOQ | Target 1-3 units for high-value items (CBCT, Chairs); 5-10 for scanners/autoclaves. Accept higher MOQ for custom OEM if NRE fees are waived. | Top 20% manufacturers now offer “pilot batch” MOQs (50% below standard) for distributors with 24-month contracts. |
| NRE & Tooling Costs | Negotiate NRE refund upon reaching annual volume thresholds (e.g., $5k NRE refunded after 10 chairs ordered). Insist on ownership of custom molds. | Chinese manufacturers increased NRE by 18% avg. in 2025 due to precision component costs (e.g., CBCT gantry). |
| Payment Terms | Push for 30% deposit, 70% against BL copy. Avoid >50% upfront payments. Escrow services recommended for first orders. | LC at sight remains standard; T/T 30 days requires credit check. 22% of 2025 disputes involved payment terms. |
Pro Tip: Bundle products (e.g., Chair + Autoclave) to meet MOQ while diversifying inventory. Distributors leveraging this saw 14% lower per-unit costs in 2025.
Step 3: Structuring Shipping Terms (DDP vs. FOB Analysis)
2026 freight volatility and new carbon regulations make Incoterms selection critical for landed cost accuracy. DDP adoption surged 37% among dental distributors in 2025.
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight/carrier. Potential savings via consolidated shipping. | Buyer bears all risk after cargo loading. Customs delays/broker fees become your liability. | Only for experienced importers with established freight partners. Avoid for first-time buyers. |
| DDP (Delivered Duty Paid) | Single all-in price. No hidden port/customs fees. Easier budgeting. | Supplier manages all logistics/risk. Critical for meeting clinic installation timelines. | STRONGLY RECOMMENDED for 90% of clinics/distributors. Reduces administrative burden by 65%. |
Key 2026 Consideration: Demand carbon-neutral shipping options. EU distributors now face 5.2% tariff surcharges for non-compliant shipments (CBAM Phase 2).
Recommended Reliable Partner: Shanghai Carejoy Medical Co., LTD
As a benchmark for compliant sourcing, Shanghai Carejoy exemplifies 2026 best practices:
- Regulatory Compliance: ISO 13485:2016 (Certificate #CN-2023-14589), CE MDR 2017/745 (NB 2797), NMPA Class II Registration (国械注准20243060085)
- MOQ Flexibility: 1-unit pilot orders for CBCT/scanners; 2 chairs for clinics. No NRE for standard OEM branding.
- Shipping: DDP standard to 45+ countries with carbon-neutral freight options. FOB available upon request.
- Verification: Real-time certificate access via compliance portal
Why 19 years in dental manufacturing matters: Their Baoshan District facility (20,000m²) maintains dedicated R&D labs for scanner/CBCT calibration, ensuring product consistency per ISO 15006:2023 standards.
Next Steps: Request Verified 2026 Price List
Shanghai Carejoy Medical Co., LTD
Baoshan District Industrial Park, Shanghai 200949, China
✉️ [email protected] | 📱 +86 15951276160 (24/7 English Support)
Reference “2026 CONSULTANT GUIDE” for priority compliance documentation and DDP landing cost analysis.
Disclaimer: This guide reflects 2026 regulatory landscapes based on current EU MDR, FDA, and Chinese NMPA trajectories. Verify all requirements with legal counsel. Shanghai Carejoy is cited as an exemplar meeting all outlined criteria; inclusion does not constitute exclusive endorsement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Subject: Frequently Asked Questions on Procuring Dental Equipment Price Lists in 2026
Frequently Asked Questions (FAQs)
- North America: 110–120V, 60Hz
- Europe, Middle East, Africa: 220–240V, 50Hz
- Asia-Pacific: 220–240V, 50Hz (varies by country)
Always confirm voltage compatibility on the equipment’s technical datasheet. Units not matching local voltage may require step-down/step-up transformers, which can affect warranty and long-term reliability. Ensure the supplier provides dual-voltage models or region-specific variants.
| Component | Standard Availability Period | Recommended Action |
|---|---|---|
| Motors, Handpieces, Valves | 10–15 years | Stock critical spares during initial rollout |
| Electronics & Control Boards | 8–10 years | Verify with distributor for legacy support |
| Consumables (e.g., O-rings, filters) | Indefinite (if model remains active) | Include in recurring supply contracts |
Request a Spare Parts Commitment Letter from the manufacturer or authorized distributor.
- Site assessment (plumbing, power, network readiness)
- Physical setup and calibration
- Software configuration and integration with clinic management systems
- Operator training (1–2 hours per unit)
Note: On-site installation is included in most premium packages but may incur additional fees for remote locations. Confirm whether installation is provided by the manufacturer, distributor, or third party—and ensure technicians are factory-certified.
- 2 years comprehensive for dental chairs, delivery systems, and imaging devices
- 1 year for handpieces and electronic components
Coverage Includes: Defects in materials, workmanship, and mechanical/electrical failures under normal use.
Common Exclusions:
| Damages from improper installation or voltage surges |
| Wear-and-tear items (e.g., chair upholstery, tubing) |
| Failure due to lack of preventive maintenance |
| Software bugs unrelated to hardware |
Extended warranty options (up to 5 years) are available for critical systems. Always obtain warranty terms in writing and verify transferability in case of resale.
- Equipment unit cost (FOB origin or delivered)
- Shipping, insurance, and import duties (if applicable)
- Installation and commissioning fees
- Training hours (if billed separately)
- Warranty extension options and pricing
- Estimated annual maintenance contract (AMC) costs
Request a total cost of ownership (TCO) breakdown for 5–7 years. Compare quotes using standardized line items and confirm if pricing is locked for 90 days. Only accept price lists bearing a valid issue date, product SKUs, and manufacturer authorization to prevent outdated or non-compliant offers.
Need a Quote for Dentale Price List?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


