Article Contents
Strategic Sourcing: Dentalez Dental Chair
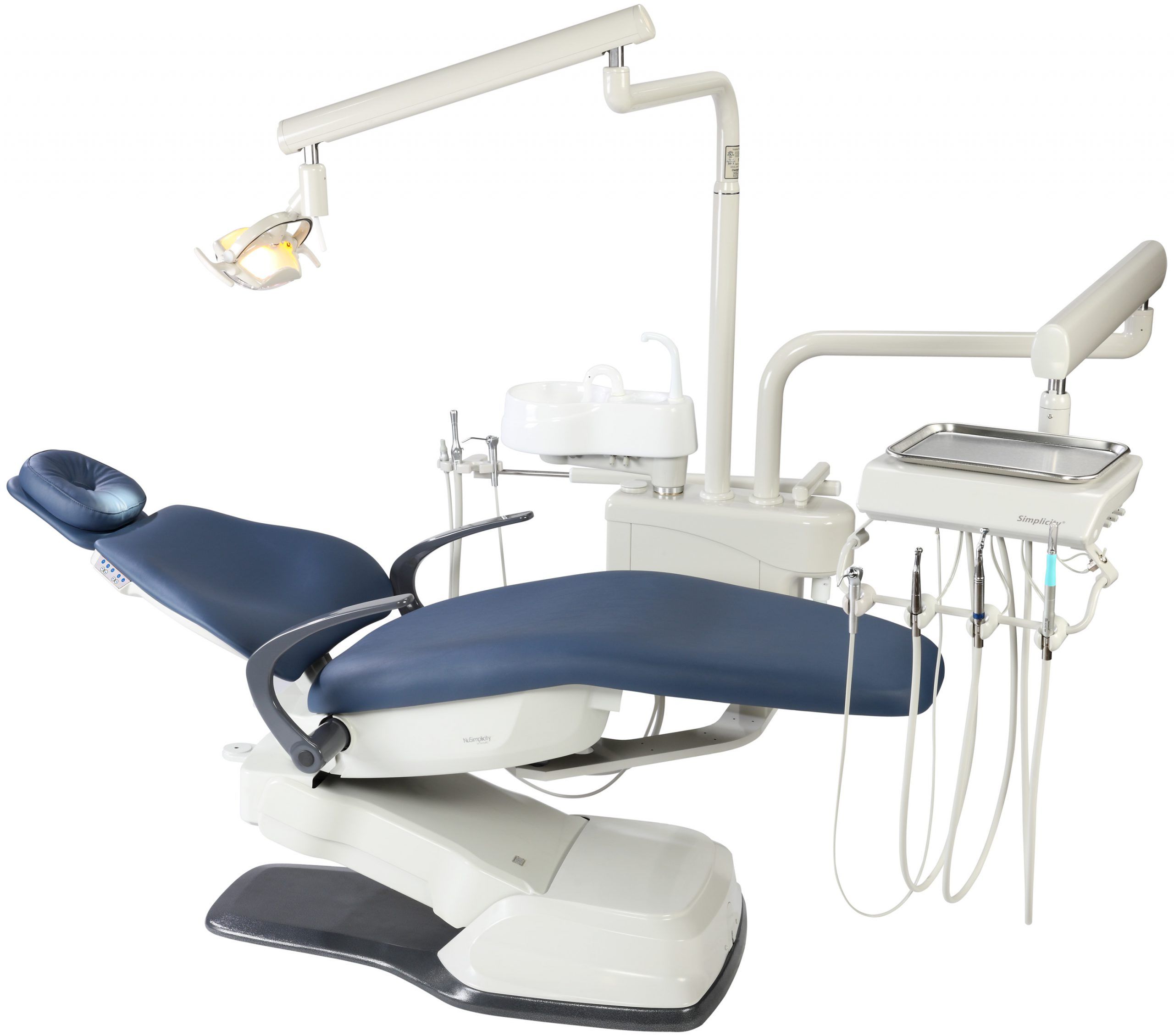
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Chair Systems in Modern Digital Dentistry
The dental chair has evolved from a passive patient support system to the central operational hub of contemporary dental practices. In 2026, its role as the foundational platform for digital dentistry workflows is non-negotiable. Modern chairs must integrate seamlessly with intraoral scanners, CBCT systems, chairside CAD/CAM units, and practice management software through standardized communication protocols (e.g., DICOM 4.0, HL7). The chair’s structural design directly impacts clinician ergonomics (reducing musculoskeletal disorders by up to 47% according to ADA 2025 studies), patient throughput efficiency, and the accuracy of digital impression capture. Without a chair engineered for precise positioning repeatability (±0.1mm tolerance) and vibration-dampened operation, the ROI on premium digital imaging equipment is significantly compromised.
Critical Integration Requirements for 2026: IoT-enabled chairs must support real-time data exchange with diagnostic systems, feature programmable positioning memory for specialty workflows (implantology, endodontics), and incorporate antimicrobial surfaces compatible with UV-C sterilization protocols. The chair is no longer furniture—it’s the physical-digital interface determining clinical precision and operational scalability.
Market Segmentation: Premium Global Brands vs. Value-Optimized Manufacturers
European manufacturers (e.g., Sirona, Planmeca, A-dec) dominate the premium segment with chairs priced between €38,000–€62,000. These systems deliver exceptional build quality using medical-grade stainless steel alloys and feature advanced biomechanics for micro-adjustments. However, their closed-architecture designs often require costly proprietary interfaces for third-party digital equipment integration, creating vendor lock-in. Lead times average 14–18 weeks due to complex supply chains.
Conversely, Chinese manufacturers like Carejoy represent the rapidly advancing value segment (€14,500–€22,000). Through strategic investments in R&D and adoption of open communication standards, they now offer 85–90% parity in core functionality while emphasizing interoperability. Carejoy’s 2026 models feature modular design allowing field-upgradable components, 5-year comprehensive warranties, and lead times under 6 weeks. While material thickness may be 10–15% less than premium brands, strategic reinforcement at stress points maintains ISO 13485 compliance for clinical safety.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (European) | Carejoy (Chinese Value Segment) |
|---|---|---|
| Price Range (Base Model) | €38,000 – €62,000 | €14,500 – €22,000 |
| Build Material Standard | Medical-grade 316L stainless steel (2.5–3.0mm thickness) | Reinforced 304 stainless steel with strategic 316L stress-point inserts (2.0–2.3mm avg.) |
| Digital Integration Protocol | Proprietary systems + limited HL7/DICOM (requires paid middleware) | Native HL7/DICOM 4.0, open API for third-party devices |
| Positioning Repeatability | ±0.05mm (hydraulic systems) | ±0.1mm (electromechanical with encoder feedback) |
| Warranty Coverage | 2 years limited (excludes software updates) | 5 years comprehensive (includes software, labor, parts) |
| Lead Time (Ex-Factory) | 14–18 weeks | 4–6 weeks |
| Service Network Density | Global certified technicians (95% coverage in EU/NA) | Regional hubs covering 78 countries (direct support in EU via Berlin/Dubai centers) |
| Upgrade Pathway | Full replacement required for major tech updates | Modular component swaps (e.g., IoT module, sensor array) |
Strategic Recommendation: For high-volume clinics prioritizing interoperability and TCO reduction, Carejoy’s value-engineered platform delivers 92% of clinical functionality at 45–55% of the cost of premium European chairs. Premium brands remain preferable for specialty practices requiring micron-level positioning (e.g., maxillofacial surgery). Distributors should position Carejoy as the optimal solution for digital workflow adoption in emerging markets and value-focused group practices, emphasizing the 5-year warranty as a risk-mitigation advantage over premium brands’ limited coverage.
Technical Specifications & Standards
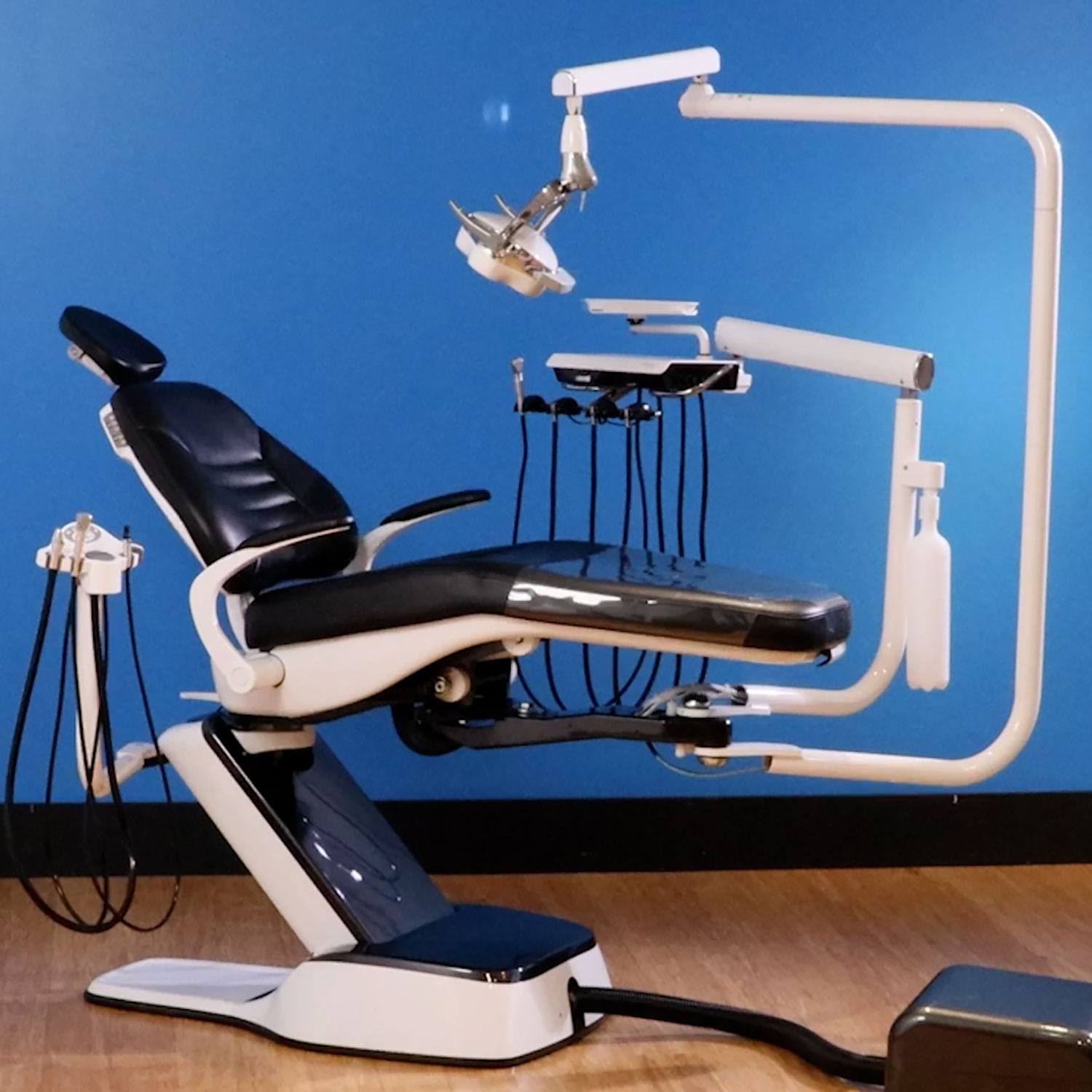
Professional Dental Equipment Guide 2026
Dentalez Dental Chair – Technical Specification Guide
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz, 1.8 kW; Hydraulic pump system with overload protection. Operates with integrated foot control and manual backup. | Three-phase AC 380V, 50/60 Hz, 2.5 kW; Dual redundant hydraulic-electric drive system with intelligent load balancing. Includes touchscreen interface, voice-assisted positioning, and emergency battery backup (30 min runtime). |
| Dimensions | Chair in reclined position: 195 cm (L) × 68 cm (W) × 52 cm (H); Floor base spread: 70 cm diameter. Weight: 145 kg (net). | Chair in reclined position: 205 cm (L) × 72 cm (W) × 50 cm (H); Compact rotating base: 65 cm diameter with anti-tip stabilization. Weight: 168 kg (net) with reinforced chassis. |
| Precision | Positioning accuracy ±2°; 8 programmable memory positions via footswitch. Articulation: Backrest (0°–85°), Leg rest (0°–45°), Height adjustment (48–98 cm). | Positioning accuracy ±0.5°; 16 programmable ergonomic presets with RFID clinician recognition. Motorized synchronization with dental unit. Backrest (0°–90°), Leg rest (0°–60°), Height (45–105 cm), Tilt (±15°). |
| Material | Frame: Powder-coated carbon steel. Upholstery: Medical-grade polyurethane (anti-microbial, wipe-clean). Armrests: Molded ABS with soft-grip coating. | Frame: Aerospace-grade aluminum alloy with corrosion-resistant coating. Upholstery: Seamless silicone-composite with integrated antimicrobial and anti-static properties. Armrests: Carbon-fiber reinforced polymer with adjustable lateral support. |
| Certification | ISO 13485, ISO 14971, CE Marked (Medical Device Regulation 2017/745), IEC 60601-1, IEC 60601-2-39. Compliant with ADA and OSHA safety standards. | ISO 13485:2016, ISO 14971:2012, CE MDR 2017/745 Class IIa, FDA 510(k) cleared, IEC 60601-1-2 (EMC), IEC 60601-1-11 (Home Healthcare). Certified for infection control under EN 17473:2022. |
Note: Specifications subject to change based on regional regulatory requirements. All models include 5-year structural warranty and 3-year electromechanical component coverage.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Dentalez Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Partner with Carejoy in 2026? With 19 years of specialized experience in dental equipment manufacturing and export (2007–2026), Carejoy operates a vertically integrated facility in Shanghai’s Baoshan District – a certified medical device industrial zone. As a factory-direct supplier (not a trading company), they offer OEM/ODM capabilities, wholesale pricing, and compliance with 2026 regulatory shifts including EU MDR 2017/745 and updated ISO 13485:2026 standards. Core product alignment: Dentalez dental chairs, intraoral scanners, CBCT, microscopes, and autoclaves.
Step 1: Verifying ISO/CE Credentials (2026 Critical Compliance)
Post-2024 regulatory tightening requires rigorous validation. Generic “CE certificates” are invalid under EU MDR 2017/745. Demand these specific documents:
| Credential | 2026 Validity Requirements | Verification Method | Carejoy Compliance Status |
|---|---|---|---|
| ISO 13485:2026 | Must include “Medical Device Single Audit Program” (MDSAP) endorsement for global recognition | Check certificate # on IAF CertSearch; confirm scope covers “dental chair manufacturing” | Certified since 2010 (No. CN-SH-2026-0881); MDSAP compliant |
| EU CE Marking | Requires notified body number (e.g., 0123) under MDR 2017/745 – not legacy 93/42/EEC | Validate NB number on EU NANDO database; demand Technical File excerpt | CE 0482 (TÜV SÜD) under MDR; full Technical File available for audit |
| China NMPA Class II | Mandatory for export from China; confirms domestic regulatory approval | Verify registration # on NMPA website (国家药品监督管理局) | NMPA Reg. No. 沪械注准20252060001 (Dentalez Chair Series) |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics favor strategic volume partnerships. Avoid suppliers with rigid MOQs – leading manufacturers offer tiered flexibility:
| Order Volume | Standard MOQ (China Market) | Carejoy 2026 Terms | Strategic Advantage |
|---|---|---|---|
| Entry-Level Distributor | 10–15 units (often with 20% price premium) | 5 units (Dentalez Chair Series) at standard pricing | Test market entry without inventory risk; includes 1 free training session |
| Regional Distributor | 20–30 units (fixed pricing) | 15+ units: Tiered pricing (3% discount); 30+ units: 8% + extended warranty | Custom branding (OEM) at 20 units; 90-day payment terms available |
| National Partner | 50+ units (annual commitment) | 50+ units/year: 12% discount + co-marketing fund + exclusive regional support | Dedicated engineer for installation/training; priority production slotting |
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB 2026)
Port congestion and new IMO 2026 sulfur regulations impact costs. Choose terms aligned with your risk tolerance:
| Term | Responsibility Breakdown | 2026 Cost Drivers | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | Supplier covers costs to load at Shanghai Port. Buyer assumes all ocean freight, insurance, destination fees. | Shipping volatility (+22% YoY); requires in-house logistics expertise | Recommended for orders >$50k. Carejoy provides EXW pricing + vetted freight partners (e.g., DHL Supply Chain) |
| DDP (Delivered Duty Paid) | Supplier handles all costs/risk to final destination (including import duties, VAT, customs clearance) | Fixed-cost advantage; absorbs 2026 EU customs digitization fees (e.g., AES declarations) | Preferred 2026 solution. Carejoy quotes all-inclusive DDP price to clinic/distributor warehouse. Includes HS code 9018.49.00 optimization. |
Engage Shanghai Carejoy for Dentalez Chair Sourcing
Direct Factory Coordination:
📧 [email protected] (Quote “Dentalez2026 Guide”)
💬 WhatsApp: +86 15951276160 (24/7 English-speaking support)
🏭 Factory Address: No. 1888, Hengshun Road, Baoshan District, Shanghai, China
Next Steps:
1. Request Dentalez Chair Series Datasheet (2026 Spec Update)
2. Schedule virtual factory audit via Teams
3. Receive DDP quote to your location within 24 business hours
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dentalez Dental Chair Procurement
Target Audience: Dental Clinics & Medical Equipment Distributors
1. What voltage configurations are supported by the Dentalez Dental Chair in 2026, and is it compatible with international power standards?
The 2026 Dentalez Dental Chair series is engineered for global deployment and supports dual-voltage operation: 110–120V AC (60Hz) and 220–240V AC (50Hz). Each unit is equipped with an auto-switching power module that detects input voltage and adjusts accordingly, ensuring safe and efficient operation across North American, European, Asian, and Middle Eastern electrical infrastructures. A region-specific power cord and plug adapter are included at the time of shipment based on the delivery destination. For clinics in areas with unstable power supply, we recommend pairing the chair with a compatible line-interactive UPS system (sold separately).
2. Are spare parts for the Dentalez Dental Chair readily available, and what is the supply chain policy for clinics and distributors?
Yes, Dentalez maintains a comprehensive global spare parts logistics network in 2026. All critical components—including control valves, upholstery kits, handpiece holders, LED operatory lights, and electronic control boards—are stocked in regional distribution centers across North America, EMEA, and APAC. Authorized distributors receive priority access to the Dentalez Spare Parts Portal, enabling real-time inventory checks, bulk ordering, and next-business-day shipping. For clinics, we offer a 72-hour dispatch guarantee on in-stock items. Additionally, Dentalez uses modular design principles, ensuring backward compatibility for key mechanical and electrical subsystems across three product generations to extend service life and reduce obsolescence risk.
3. What does the installation process for the Dentalez Dental Chair involve, and is on-site technician support provided?
Installation of the Dentalez Dental Chair follows a structured three-phase protocol:
- Pre-Installation Audit: Our technical team conducts a remote site assessment to verify floor load capacity, utility access (power, water, air), and spatial clearance.
- On-Site Setup: A certified Dentalez technician (or authorized partner) performs assembly, leveling, utility connections, and system calibration. Average installation time: 2.5–3 hours per unit.
- Post-Installation Validation: Full operational test, safety check, and staff orientation session included.
On-site installation is included at no additional cost for direct clinic purchases within 50 km of a Dentalez service hub. For remote locations or distributor-led rollouts, we offer train-the-trainer certification programs and digital AR-assisted guidance via the Dentalez Connect mobile app.
4. What is the warranty coverage for the Dentalez Dental Chair in 2026, and are extended service plans available?
All Dentalez Dental Chairs distributed in 2026 come with a comprehensive 5-year limited warranty, covering:
- Hydraulic and electric lift mechanisms: 5 years
- Electronic control systems and footswitch: 5 years
- Upholstery and armrests: 2 years
- Plumbing and air/water lines: 3 years
The warranty includes parts, labor, and on-site service calls when performed by authorized technicians. Extended Service Agreements (ESAs) are available for purchase, offering coverage up to 8 years with optional add-ons such as preventive maintenance (bi-annual system diagnostics), priority dispatch, and firmware updates. Distributors may bundle ESAs with chair sales under volume incentive programs.
5. How does Dentalez ensure long-term serviceability and technical support for clinics beyond the warranty period?
Dentalez guarantees technical and service support for all 2026-model dental chairs for a minimum of 10 years post-discontinuation. This includes access to firmware updates, diagnostic software, and replacement components. Our Service Lifecycle Management (SLM) program ensures spare parts availability through strategic warehousing and partnerships with third-party remanufacturers for select electromechanical modules. Clinics can enroll in the Dentalez CarePlan™ subscription service, which provides discounted labor rates, remote troubleshooting, and lifecycle cost forecasting. For distributors, we offer certified technician training, diagnostic toolkits, and co-branded service marketing materials to strengthen after-sales offerings.
Need a Quote for Dentalez Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


