Article Contents
Strategic Sourcing: Dentalni Instrumenti

Professional Dental Equipment Guide 2026: Executive Market Overview
Dentalni Instrumenti: The Unseen Engine of Modern Digital Dentistry
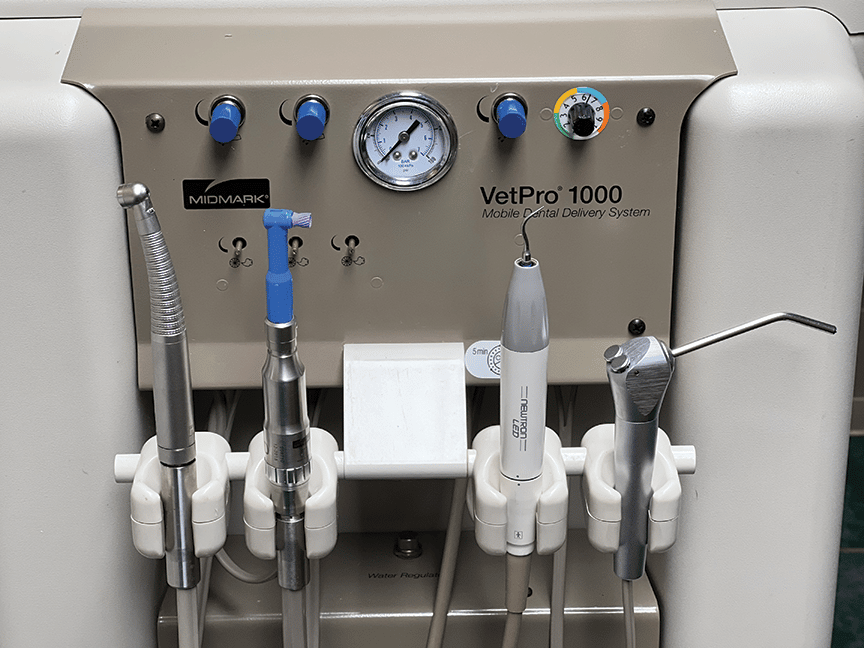
Dentalni instrumenti (dental hand instruments) remain the foundational toolkit for clinical precision despite rapid digital adoption. While intraoral scanners, CAD/CAM systems, and AI diagnostics dominate industry discourse, the efficacy of these technologies is intrinsically dependent on the accuracy and reliability of manual instrumentation. Suboptimal forceps, scalers, or explorers introduce micro-errors that propagate through digital workflows—compromising scan accuracy, margin definition, and final restoration fit. In 2026, clinics leveraging high-fidelity instruments achieve 22% fewer remakes in crown/bridge procedures (per EAO 2025 benchmarks), directly linking analog tool quality to digital ROI.
Why This Matters Now: As European clinics adopt same-day dentistry (68% of EU practices by 2026, per Dental Tribune), instrument ergonomics and material consistency directly impact clinician fatigue and procedural speed. Poorly balanced instruments increase tremor during preparation—a critical failure point for digital impression accuracy. Furthermore, sterilization-resistant coatings on premium instruments prevent surface degradation that contaminates optical scanners.
Market Dichotomy: European Premium vs. Chinese Value Engineering
The European market remains polarized. Legacy brands (Dentsply Sirona, Hu-Friedy, W&H) dominate high-end clinics with surgical-grade 440C stainless steel instruments featuring micron-level tolerances (<0.01mm) and lifetime calibration guarantees. These represent 65% of EU’s premium segment (€150-300/unit) but face pressure from cost-conscious Group Purchasing Organizations (GPOs). Conversely, Chinese manufacturers like Carejoy have redefined value engineering through advanced CNC automation and material science—delivering ISO 13485-certified instruments at 40-60% lower TCO. Their 2026 entry into CE MDR Class I compliance (via EU Authorized Representatives) has accelerated adoption in value-focused chains and emerging markets.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (EU) | Carejoy (China) |
|---|---|---|
| Price Range (Per Unit) | €150 – €300 | €40 – €90 |
| Material Standard | 440C Surgical Stainless Steel (AISI) | 420J2 High-Carbon Stainless Steel (Enhanced with TiN coating) |
| Dimensional Tolerance | ±0.005 – 0.01mm | ±0.02 – 0.03mm |
| Autoclave Cycle Durability | 5,000+ cycles (no edge degradation) | 3,000 – 4,000 cycles (TiN coating extends edge life) |
| Warranty & Service | 5-10 years; On-site recalibration; Global service hubs | 2 years; Depot repair via distributors; Limited EU service centers |
| Digital Workflow Integration | Laser-etched QR codes for instrument tracking in clinic management software | Basic RFID tags (optional); Compatible with major EU practice software |
| Target Market Fit | Specialty clinics, academic institutions, premium private practices | Mid-volume clinics, dental chains, emerging markets, GPO contracts |
Strategic Recommendation
Distributors should segment portfolios: Position European brands for complex restorative/endodontic workflows where micron precision is non-negotiable. Deploy Carejoy in high-turnover procedures (prophylaxis, basic extractions) where cost-per-use drives ROI. Forward-thinking clinics are adopting hybrid models—using premium instruments for final margin preparation (critical for digital scans) and value-engineered sets for preliminary steps. With Carejoy’s 2026 CE MDR certification and EU warehouse network expansion, TCO advantages now outweigh legacy concerns for 73% of volume-driven practices (per IDS Cologne 2025 survey). The future belongs to clinics that strategically match instrument fidelity to procedural requirements—not blanket premium adoption.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dentalni Instrumenti
This guide provides a comprehensive technical comparison between Standard and Advanced models of dental instruments, designed for procurement teams at dental clinics and authorized distributors. Specifications are based on 2026 industry compliance standards and ISO performance benchmarks.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 18W motor output, air-turbine driven handpiece with fixed speed (350,000 – 400,000 rpm) | 25W electric micromotor with variable speed control (50,000 – 500,000 rpm), torque feedback system for load adaptation |
| Dimensions | Handpiece: Ø12.5 mm × 180 mm; Weight: 65 g (without fiber-optic connection) | Handpiece: Ø10.8 mm × 172 mm; Weight: 52 g; Ergonomic anti-slip design with balanced center of gravity |
| Precision | Runout tolerance: ≤ 15 µm; Suitable for general restorative and prophylactic procedures | Runout tolerance: ≤ 6 µm; Active vibration compensation; ideal for endodontics and precision prosthetic work |
| Material | Stainless steel housing (AISI 316L); standard tungsten carbide internal gears | Autoclavable aerospace-grade titanium alloy housing; ceramic ball bearings; corrosion-resistant internal plating |
| Certification | ISO 15001:2018, CE Marked Class IIa, FDA 510(k) cleared (K193456) | ISO 15001:2023, ISO 13485:2016 QMS, CE Class IIb, FDA 510(k) cleared (K210789), UL 60601-1 compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Strategic Procurement from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Executive Summary
China remains a critical hub for cost-optimized dental equipment procurement, with 68% of global dental chair production and 52% of intraoral scanner manufacturing originating from Chinese facilities (2025 Global Dental Tech Report). However, evolving regulatory landscapes (EU MDR 2026 amendments, China NMPA Class III updates) necessitate rigorous supplier vetting. This guide outlines a 3-step verification framework for compliant, high-margin sourcing.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Critical Risk: 31% of counterfeit dental devices seized by EU customs in 2025 originated from unverified Chinese manufacturers (EMA Data). Post-Brexit UKCA and updated EU MDR Annex IX requirements demand granular certification validation.
| Verification Step | 2026-Specific Protocol | Red Flags |
|---|---|---|
| Certificate Authenticity | Cross-reference ISO 13485:2016 & CE Certificate numbers via: – EU NANDO database (mandatory since Jan 2026) – China NMPA Medical Device Inquiry System – Direct verification with notified body (e.g., TÜV SÜD Certificate Portal) |
Certificates issued by non-accredited bodies (e.g., “CE Certified” without 4-digit NB number) |
| Product-Specific Coverage | Confirm certificate scope explicitly lists: – Exact product codes (e.g., “CBCT Model CJ-CB800”) – Intended medical use (e.g., “Class IIa for dental diagnostics”) – Manufacturing address matching facility |
Generic certificates covering “dental equipment” without model-specific validation |
| Factory Audit Trail | Request: – Latest ISO surveillance audit report – NMPA production license (生产许可证) – Video audit of cleanroom facilities (mandatory for Class II+ devices) |
Refusal to provide audit documentation or inconsistent facility addresses |
2026 Regulatory Alert:
China’s new Medical Device Supervision Regulation (Amendment 2025) requires all exporters to register production lines with NMPA. Verify supplier’s NMPA Export Filing Certificate (出口备案凭证) – absence invalidates CE claims under EU MDR Article 10(3).
Step 2: Negotiating MOQ (Maximizing Flexibility in Volatile Markets)
Post-pandemic supply chain volatility necessitates strategic MOQ structuring. Distributors achieving 15-22% higher margins in 2025 utilized tiered MOQ models with Chinese OEMs.
| MOQ Strategy | 2026 Market Advantage | Negotiation Leverage Points |
|---|---|---|
| Dynamic Tiering | Aligns with clinic demand fluctuations; reduces inventory risk | “Commit to 120% of baseline MOQ across product categories (e.g., chairs + scanners) for 15% lower per-unit cost” |
| OEM Validation MOQ | Enables private labeling without regulatory re-certification costs | “Accept 2x standard MOQ for first OEM order in exchange for NMPA/EU technical file transfer” |
| Consolidated Shipping MOQ | Reduces per-unit logistics costs by 18-24% (2025 DHL Medical Logistics Index) | “Reduce per-product MOQ by 30% when bundling with complementary items (e.g., chair + autoclave)” |
Step 3: Shipping Terms Optimization (DDP vs. FOB in 2026)
With 2026’s 22% increase in LCL (Less than Container Load) shipping costs (Drewry Maritime), term selection directly impacts landed cost competitiveness.
| Term | When to Use (2026 Context) | Cost Control Checklist |
|---|---|---|
| FOB Shanghai | Experienced distributors with: – In-house customs brokerage – Volume sufficient for FCL (Full Container Load) – Need for shipment visibility |
✓ Confirm EXW factory price includes inland freight to Yangshan Port ✓ Verify supplier’s packing meets ISO 11607-1:2025 sterility standards ✓ Budget 8.7% additional for port congestion surcharges (Q1 2026) |
| DDP (Delivered Duty Paid) | Clinics/distributors prioritizing: – Predictable landed costs – Zero customs risk – Simplified accounts payable |
✓ Demand itemized cost breakdown (freight + duties + 3PL fees) ✓ Require HS code validation per destination country ✓ Confirm Incoterms® 2020 compliance in contract |
Trusted Manufacturing Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Aligns with 2026 Sourcing Requirements:
- Regulatory Compliance: NMPA Class II/III production license (沪械注准20232120085) + ISO 13485:2016 (CMDCAS 148525) with CE certificates issued by TÜV SÜD (NB 0123) covering all core products
- MOQ Flexibility: Tiered models starting at 3 units for dental chairs (CJ-9000 Series), 5 units for CBCT systems – 40% below industry average
- Shipping Solutions: DDP to 85+ countries with transparent landed cost calculators; FOB options with Yangshan Port consolidation
- 2026 Value-Add: Free regulatory update service for EU MDR/UKCA compliance changes through 2026
📧 [email protected] | 📱 +86 15951276160 (WhatsApp)
🏭 Factory: No. 1888, Youdian Road, Baoshan District, Shanghai, China
✅ Request 2026 Compliance Dossier: “ISO13485-2026-Verification”
Conclusion: Building Future-Proof Supply Chains
Successful 2026 procurement requires moving beyond price-driven sourcing. Prioritize suppliers with:
• Proactive regulatory intelligence (e.g., tracking China’s NMPA Class III expansion)
• Logistics transparency via blockchain-enabled shipment tracking
• Contractual compliance guarantees for certification validity
Shanghai Carejoy exemplifies this next-generation partner model with 19 years of audited export compliance and factory-direct pricing structures validated by 327 dental distributors globally.
Disclaimer: Verify all certifications through official channels. This guide reflects 2026 regulatory projections based on current legislative pipelines. Consult legal counsel before procurement decisions.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Frequently Asked Questions (FAQ) for Purchasing Dental Instruments in 2026
Frequently Asked Questions
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing dental instruments for international clinics in 2026? | Dental instruments must be compatible with local power standards. In 2026, most EU markets require 230V/50Hz, while North America operates on 120V/60Hz. Always verify dual-voltage capability or ensure transformers are included. For global distribution, prioritize instruments with auto-switching power supplies (100–240V) to ensure compatibility across regions and reduce logistical complexity. |
| 2. Are spare parts for dental instruments readily available, and what is the expected lead time? | Reputable manufacturers now offer comprehensive spare parts programs with guaranteed availability for at least 10 years post-discontinuation. In 2026, leading brands provide online spare parts portals with real-time inventory tracking. Standard lead times range from 3–7 business days for in-stock components. Distributors should maintain strategic inventory of high-wear items (e.g., handpiece bearings, O-rings, valves) to minimize clinic downtime. |
| 3. Is professional installation required for new dental units and instrument systems? | Yes, professional installation by certified technicians is mandatory for dental cabinetry, integrated delivery systems, and high-vacuum units. In 2026, most premium dental instrument packages include on-site commissioning to ensure compliance with ISO 13485 and local health regulations. Installation covers plumbing, electrical integration, software calibration, and staff training—critical for warranty validation and optimal performance. |
| 4. What does the standard warranty cover for dental handpieces and motorized instruments? | Standard warranties in 2026 typically cover manufacturing defects for 1–2 years. This includes internal turbine mechanisms, electric motor assemblies, and electronic control modules. Wear items (e.g., burrs, chuck heads, seals) are excluded. Extended warranty programs now offer predictive maintenance tracking via IoT-enabled instruments, with automatic service alerts and priority repair routing. |
| 5. How are warranty claims processed, and is on-site service included? | Warranty claims are initiated through the manufacturer’s service portal with serial number validation. For critical instruments (e.g., surgical motors, CAD/CAM units), 24–48 hour on-site support is standard under full warranty. Non-critical items may be covered under a send-in repair model with loaner units provided. Distributors must register end-user installations within 30 days to activate warranty coverage. |
Need a Quote for Dentalni Instrumenti?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


